Mutations in α- and β-tubulin encoding genes: Implications in brain malformations
Transcript of Mutations in α- and β-tubulin encoding genes: Implications in brain malformations

www.elsevier.com/locate/braindev
Brain & Development xxx (2014) xxx–xxx
Review article
Mutations in a- and b-tubulin encoding genes: Implicationsin brain malformations
Romina Romaniello a, Filippo Arrigoni b, Maria Teresa Bassi c, Renato Borgatti a,⇑
a Neuropsychiatry and Neurorehabilitation Unit, IRCCS Eugenio Medea, Bosisio Parini, Lecco, Italyb Neuroimaging Unit, IRCCS Eugenio Medea, Bosisio Parini, Lecco, Italy
c Laboratory of Molecular Biology, IRCCS Eugenio Medea, Bosisio Parini, Lecco, Italy
Received 25 March 2014; received in revised form 26 May 2014; accepted 2 June 2014
Abstract
The tubulin gene family is mainly expressed in post-mitotic neurons during cortical development with a specific spatial and temporalexpression pattern. Members of this family encode dimeric proteins consisting of two closely related subunits (a and b), representingthe major constituents of microtubules. Tubulin genes play a crucial role in the mechanisms of the Central Nervous System develop-ment such as neuronal migration and axonal guidance (axon outgrowth and maintenance). Different mutations in a/b-tubulin genes(TUBA1A, TUBA8, TUBB2A, TUBB4A, TUBB2B, TUBB3, and TUBB) might alter the dynamic properties and functions of micro-tubules in several ways, effecting a reduction in the number of functional tubulin heterodimers and causing alterations in GTP bindingand disruptions of the binding of other proteins to microtubules (motor proteins and other microtubule interacting proteins).
In recent years an increasing number of brain malformations has been associated with mutations in tubulin genes: malformationsof cortical development such as lissencephaly and various grades of gyral disorganization, focal or diffuse polymicrogyria and openor closed-lips schizencephaly as likely consequences of an altered neuronal migration process; abnormalities or agenesis of themidline commissural structures (anterior commissure, corpus callosum and fornix), hypoplasia of the oculomotor and optic nerves,dysmorphisms of the hind-brain as expression of axon guidance disorders. Dysmorphisms of the basal ganglia (fusion between thecaudate nucleus and putamen with absence of the anterior limb of the internal capsule) and hippocampi were also observed. A rareform of leukoencephalopathy characterized by hypomyelination with atrophy of the basal ganglia an cerebellum (H-ABC) was alsorecently described.
The present review, describing the structural and functional features of tubulin genes, aims to revise the main cerebral associatedmalformations and related clinical aspects, suggesting a genotype–phenotype correlation.� 2014 The Japanese Society of Child Neurology. Published by Elsevier B.V. All rights reserved.
Keywords: Brain malformations; Malformations of cortical development; Tubulin genes; Genotype–phenotype correlation; Mental retardation;Epilepsy
1. Introduction
The development of the Central Nervous System(CNS) is a complex event that requires precise
http://dx.doi.org/10.1016/j.braindev.2014.06.002
0387-7604/� 2014 The Japanese Society of Child Neurology. Published by E
⇑ Corresponding author. Address: Neuropsychiatry and NeurorehabilitatioParini, Lecco, Italy. Tel.: +39 031877111; fax: +39 031877499.
E-mail address: [email protected] (R. Borgatti).
Please cite this article in press as: Romaniello R et al. Mutations in a- aBrain Dev (2014), http://dx.doi.org/10.1016/j.braindev.2014.06.002
regulation of a massive amount of cell division andmigration within a specific spatial and temporal win-dow. Neurons and glial cells must proliferate, migrateto appropriate areas in the cerebral cortex and project
lsevier B.V. All rights reserved.
n Unit, IRCCS Eugenio Medea, Via D. L. Monza 20, 23842 Bosisio
nd b-tubulin encoding genes: Implications in brain malformations.

Fig. 1. Structure of a- and b-tubulin protein: domains and functions.The structure of the a- and b-tubulin protein is schematicallyrepresented. N-terminal domain with the GTP binding pocket (N-siteand E-site): it is essential for protein folding, heterodimer stability andmicrotubule dynamics. Intermediate domain mediates longitudinalinteractions, through a series of highly conserved residues and lateralprotofilament interactions. C-terminal domain mediates the MAPs andmotor protein interactions on the external surface of tubulin.
2 R. Romaniello et al. / Brain & Development xxx (2014) xxx–xxx
multiple cellular extensions (axons and dendrites) toform synapses, thereby allowing communication withother neurons and the creation of networks [1].Although several organelle mechanisms are implicatedin these complex processes, involving cilia and centro-somes, a key role is played by microtubules (MTs) [2].
Microtubules serve essential cellular functions whichare critical for the development and maintenance ofthe CNS. They provide structure and generate forcesneeded by neurons to migrate and develop axonal anddendritic processes, providing organized scaffolds formotor proteins (like kinesin and dynein).
In recent years, mutations in genes regulating build-ing blocks, such as the a- and b-tubulin isotypes, havebeen shown to alter microtubule functions in humansand cause a wide range of neurological disorders [3–8].The family of tubulin genes is mainly expressed inpost-mitotic neurons during cortical development witha specific spatial and temporal expression pattern, play-ing a crucial role in mechanisms such as neuronal migra-tion and axonal guidance (axon outgrowth andmaintenance) [7,9–12]. Mutations of the a- and b-tubu-lin genes (TUBA1A, TUBA8, TUBB2A, TUBB4A,
TUBB2B, TUBB3, TUBB) might alter the dynamicproperties and functions of microtubules in several waysand were thus linked to multiple and complex disordersof brain development, characterized by: a broadspectrum of malformations of cortical development(MCDs), agenesis or hypoplasia of commissural axontracts and a rare form of leukoencephalopathy[3,4,6,7,13–18].
Besides describing the structural and functional fea-tures of tubulin proteins, the present review aims torevise the main cerebral associated abnormalities andrelated clinical aspects suggesting a genotype–phenotypecorrelation.
2. a- and b-tubulin proteins: structural and functionalfeatures
The a and b tubulins, which share a high degree ofhomology, form heterodimers that are incorporated intomicrotubules. Tubulins comprise three separate struc-tural domains (the N-terminal, intermediate, and C-ter-minal domains), formed by b-sheets alternated to a-helices that serve at least five functions [6,10,12,19].The N-terminal structural domain forms the GTP bind-ing pocket required for protein folding and stability andfor the correct conformation of longitudinal protofila-ments. N-terminal domain interactions with the adja-cent intermediate domain contribute to structuralrearrangements resulting from the hydrolysis of GTPbound by b-tubulin and leading to microtubule depoly-merization [6,10,12]. Other residues within this domainmediate longitudinal and lateral interactions, flankingthe side and inner surface of heterodimers that are
Please cite this article in press as: Romaniello R et al. Mutations in a- aBrain Dev (2014), http://dx.doi.org/10.1016/j.braindev.2014.06.002
necessary for both heterodimer and microtubule stabil-ity. Finally, interactions between motor proteins andmicrotubule-associated proteins (MAPs) occur throughresidues at the C-terminus that form a helices on theexternal surface of tubulin. These residues mediate inter-actions with kinesin and dynein regulate the dynamicbehavior of microtubules and are necessary for neuronmigration, differentiation and axon guidance. [10,12,19].
In higher eukaryotes, tubulins are encoded by a fam-ily of genes that are evolutionary conserved among dif-ferent species, mainly expressed in post-mitotic neuronsmostly during neuronal migration and differentiation[19]. A possible explanation for the need for these highlyconserved multiple genes is that the different isotypesmay be required to form specific sets of microtubulesthat carry out unique functions [9]. The microtubule net-work has highly specialized roles within neurons, espe-cially during cerebral development [9]. The disruptionof microtubule-based processes can lead to an impair-ment in neuronal migration and axon tract formation,resulting in a wide spectrum of brain malformations.Neuronal migration may indeed require increasedmicrotubule stability, whereas axonal growth mayrequire more dynamic microtubules in the growthcone [20]. Moreover, it has been demonstrated thatmutations in microtubule-associated genes encodingfor microtubule-associated proteins (MAPs) such asLIS1 (Lissencephaly 1) and DCX (Doublecortin),and actin cytoskeleton components such as FLNA
(Filamin A) can cause neuronal migration disorders[1]. Fig. 1 schematically represents the structure ofa and b-tubulin proteins summarizing their domainsand functions.
nd b-tubulin encoding genes: Implications in brain malformations.

R. Romaniello et al. / Brain & Development xxx (2014) xxx–xxx 3
3. a- and b-tubulin genes: mutations and functional
consequences on CNS development
Disease-causing amino acid substitutions in tubulingenes are widely distributed among the three domainsof these proteins, perturbing dynamic properties andfunctions of microtubules, according to their structurallocations. Several mutations alter residues that interactdirectly with the GTP nucleotide or those locateddirectly adjacent to it. As lateral interactions, locatednear residues that flank the side and inner surface ofheterodimers, are also important for interacting and reg-ulating microtubule dynamics following GTP hydroly-sis. Other mutations are located at contact surfacesbetween the intra-heterodimer and inter-heterodimerand thus are predicted to alter longitudinal protofila-ment interactions [9,10]. Another subset of mutationsaffects residues that lie in proximity to the interfacebetween the N-terminal and intermediate domains.These amino acid substitutions may affect heterodimerstability and/or the dynamic properties of microtubulesby altering the structure of tubulin during nucleotideexchange and hydrolysis. These types of mutationscause a wide range of gyral malformations, suggestingthat microtubule stability may be diminished duringkey processes of cell migration [9]. Finally, many substi-tutions change residues on the surface of microtubulesthat mediate protein interactions with kinesin, dyneinand other MAPs. These are associated with axonguidance and maintenance defects and oculomotordisorders.
As mutations in tubulin genes produce different phe-notypes, in a recent study Cushion et al., correlated thelocation of several mutated proteins to the sites of thea- and b-tubulin interface; these are indeed consideredimportant for heterodimer formation and stability. Bothof them are crucial for rapid changes in the cytoskeleton(e.g. axonal path finding) of the developing neuron [21].The disruption of tubulin heterodimerization seems toplay a key role in aberrant microtubule polymerization,even if two mutations (L286F in TUBA1A and V353I inTUBB without heterodimerization in vitro) have beendescribed [11,14].
Therefore, although the strong correlation betweenmutation’ effects and related clinical features is stillunder debate, the crucial role of mutations mainly inter-fering with heterodimerization in axonal pathfindingmechanisms of developing neurons has been widelydescribed [17].
An interesting line of research in future could beaimed at verifying whether other tubulin-related causesof MCDs are associated with mutations near bindingsites of other MAPs [1].
Over the past few years, mutations in neuronallyexpressed a- and b-tubulin genes (TUBA1A, TUBA8,
TUBB2A, TUBB4A, TUBB2B, TUBB3, TUBB) have
Please cite this article in press as: Romaniello R et al. Mutations in a- aBrain Dev (2014), http://dx.doi.org/10.1016/j.braindev.2014.06.002
been identified in about a hundred patients sufferingfrom a wide spectrum of brain malformations such aslissencephaly, polymicrogyria, and various grades ofgyral disorganization usually associated with additionalcerebral anomalies including callosal hypoplasia oragenesis, abnormal basal ganglia and cerebellar hypo-plasia [3,4,6,7,13–18]. The only mutation reported inthe TUBA8 gene is a 14-base pair deletion observed intwo families. The presence of the same deletion andthe concordant homozygosis of the results of SNP geno-typing and microsatellite markers analyses strongly sug-gest these families are related (as explicitly stated by theauthors) [3]. Moreover, it has been reasonably well dem-onstrated that the TUBA8 gene is expressed at very lowlevels in the developing brain in mice and humans, rais-ing the possibility that it is not pathogenic at all [23].Three different de novo mutations have been identifiedin the TUBB gene in three unrelated families with micro-cephaly, suggesting the pathogenic role played by thisgene expressed at high levels in the developing brain[14]. Mutations in the TUBB4A gene have been associ-ated with a rare form of leukoencephalopathy character-ized by hypomyelination with atrophy of the basalganglia and cerebellum (H-ABC) [15–18]. This evidencesupports the hypothesis that, despite their high sequencehomology, tubulin genes have subtle but distinct roles inmicrotubular dynamics and function, according to celltype and neurodevelopmental time points [19].
4. Spectrum of the tubulin-related disorders: brainabnormalities
4.1. Malformations of cortical development
Early studies on mutations in the a- and b-tubulingenes described MCDs involving migration or post-migration defects [4].
Various degrees of lissencephaly, ranging from com-plete loss of gyri and sulci (agyria) to brain withsimplified abnormally thick convolutions (pachygyria)and classical lissencephaly have been associatedwith mutations in the Tubulin a-1A (TUBA1A) gene(ORPHA171680, OMIM 611603) [6,24]. Bilateral asym-metric polymicrogyria (PMG) was observed in associa-tion with mutations in the Tubulin b-2B (TUBB2B)gene (ORPHA300573, OMIM 610031) [4], and a muta-tion in Tubulin a-8 (TUBA8) (ORPHA250972, OMIM613180) was described in subjects with generalizedPMG and optic nerve hypoplasia [3].
Subsequently, the spectrum of cortical malformationsassociated with a-and b-tubulin gene mutations hasbroadened: perisylvian polymicrogyria (PMG) andPMG-like patterns have been associated with mutationsin TUBA1A [25-35]; various types of lissencephaly aswell as more complex gyral disorganization and schizen-cephaly (SCH), congenital fibrosis of extraocular
nd b-tubulin encoding genes: Implications in brain malformations.

4 R. Romaniello et al. / Brain & Development xxx (2014) xxx–xxx
muscles, and axon dysinnervation were found in subjectscarrying TUBB2B gene defects [36–39]. PMG and sim-plified and disorganized gyral patterning were describedin subjects with mutations in the Tubulin b-3 (TUBB3)gene (ORPHA300570, OMIM 614039) [7,40]. Neverthe-less, this gene was initially mainly described in axonguidance disorders (hypoplasia of oculomotor nervesand congenital fibrosis of extraocular muscles) [8]. Morerecently, mutations in the Tubulin b (TUBB) gene(OMIM 191130) have been described in three unrelatedcases, one of them showing focal PMG associated with
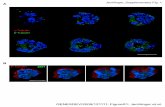
Fig. 2. Malformations of cortical development associated with muta-tions in tubulin genes. (A, B) Axial and coronal T2-weighted sectionsin a patient carrying a c.1160C>T, (p.Ala387Val) mutation inTUBA1A gene show a pachygyric cortex and subcortical bandheterotopia (black arrows) [42]. (C, D) Axial T1-weighted sections ina patient with a c.1080 1084del CCTGAinsACATCTTC (p.Leu361Lys362delinsHisLeuGln) mutation in TUBB2B gene show a simplifiedgyral pattern and a small area of heterotopic neuron in the subcorticalwhite matter (black arrowhead). Lateral ventricles are enlarged anddysmorphic [42]. (E–H) Ti-weighted sections in two different subjectswith c.419G>C (p. Gly140Ala) and c.1060T>C, p.C354R mutations inTUBB2B gene demonstrate a similar pattern characterized by open-lipschizencephaly (arrows in E–G) associated with thick polymicrogyriccortex in the surrounding and contralateral hemisphere (arrowheads inE–H) ([38] and unpublished case).
Please cite this article in press as: Romaniello R et al. Mutations in a- aBrain Dev (2014), http://dx.doi.org/10.1016/j.braindev.2014.06.002
localized band heterotopia [13]. Mutations in the Tubu-lin, b-2A (TUBB2A) gene (OMIM 615101) weredescribed in two unrelated individuals with simplifiedgyral patterning and infantile-onset epilepsy [14].
The causes of the asymmetric cerebral involvementfrequently observed in tubulin-related disorders are stillunexplained: some authors proposed that the prevalentinvolvement of the left hemisphere could be related toa different left–right expression of these genes. Accord-ing to recent studies, the concept of an asymmetricalmolecular patterning of the developmental brain hasto be extended to genes acting downstream in morpho-genesis and transcription factors [9].
Fig. 2(A–D) shows an MRI scan of the main MCDpatterns associated with a- and b-tubulin genemutations.
4.2. Midline commissural structures, basal ganglia,
cerebellum and hind-brain
Many different brain malformations have been asso-ciated with mutations in the a- and b-tubulin genes. Thishas led to the hypothesis that there is a spectrum of tub-ulinopathies ranging from abnormalities in neuronalproliferation to migration defects and disturbances inaxonal guidance [20]. Supratentorial malformationsaffect basal ganglia that often appear dysmorphic with
Fig. 3. Possible brain findings, other than MCDs, in patients withmutations in tubulin genes. In patients with mutations of tubulin genes[38,42], basal ganglia are usually dysmorphic with a fusion between thehead of the caudate nucleus and the putamen and an incompleterepresentation of the anterior limb of the internal capsule (arrow in A).The corpus callosum can be dysmorphic (B, C) or even absent (arrowin E). The anterior commissure can be absent (B) or hypoplasic(arrowhead in C). Also, the brainstem can show an irregularsegmentation (B, C). Nerve hypoplasia can be observed at multiplelevels such as optic nerves (D) and oculomotor nerves (arrowhead in Epoints at a normal III cranial nerve on the left side of the brain; theright nerve cannot be identified because it is markedly blurred). Thecerebellum can show dysplasia with an abnormal, irregular orientationof folia, as shown in D. The hippocampi can have a simplified pattern(arrows in F).
nd b-tubulin encoding genes: Implications in brain malformations.

Table 1Clinical features associated with mutations in a- and b-tubulin encoding genes.
Gene and references TUBA1A genea TUBA8 geneb TUBB2B genec TUBB3 gened TUBB genef TUBB2A geneg TUBB4A geneh
N. Patients 42 4 18 17 plus 120e 3 2 75Congenitalmicrocephaly
Yes No NA Yes No Yes No NA Yes No NA Yes No Yes No Yes No NA
18 7 17 1 3 14 1 3 4 13 3 0 0 2 43 22 10Epilepsy Yes No NA Yes No Yes No NA Yes No NA Yes No Yes No Yes No NA
16 5 21 4 0 11 7 0 3 7 7 0 3 2 0 32 39 4Intellectualdisabilityand motorimpairment
Yes No NA Yes No Yes No NA Yes No NA Yes No Yes No Yes No NA
21 0 21 4 0 18 0 0 15 0 2 3 0 2 0 All presentwith progressivedeterioration
Severe: 17 All Severe Severe: 13 Severe: 10 Severe: 1/3 All SevereModerate: 3 Moderate: 4 Moderate: 2 Moderate: 2/3Mild: 1 Mild: 1 Mild: 3
Ocularfindings
Yes No NA Yes No Yes No NA Yes No NA Yes No Yes No Yes No NA
3 2 27 4 0 9 3 6 15 2 0 1 2 0 2 19 10 463 Strabismus All OH Strabismus 2 Ptosis/CFEOM: 8 Retina dysplasia
and micro-opthalmiaNystagmus: 10
1 OH 1 OA Ptosis/CFEOM: 4 Strabismus: 15 OculomotorOA: 3 Nystagmus: 4 abnormalities:17
Abbreviations: CFEOM: congenital fibrosis of the extraocular muscles; N: number; NA: not available; OA: optic atrophy; OH: optic (nerve) ypoplasia.References: aTUBA1A gene: Cushion et al. [21]; Hikita et al. [32]; Jansen et al. [29]; Kumar et al. [6]; Mokanszki et al. [30]; Morris-Rosendahl et l. [27]; Okumura et al. [33]; Poirier et al. [24]; Poireret al. [7]; Sohal et al. [31]. bTUBA8 gene: Abdollahi et al. [3]. cTUBB2B gene: Amrom et al. [41]; Cederquist et al. [36]; Cushion et al. [21]; Gue ini et al. [37]; Jaglin et al. [4]; Romaniello et al. [38].dTUBB3 gene: Chew et al. [22]; Poirier, et al. [7]. eTischfield et al. [8] (describe 120 patients in 10 pedigrees carrying 8 TUBB3 gene mutations. No data was reported about epilepsy and microcephaly.CFEOM is present in all patients. Mild intellectual disability and mild motor impairment are present only in 30 and 18 patients, respectively) fTUBB gene: Breuss et al. [14]. gTUBB2A: Cushionet al. [13]. hTUBB4A: Only H-ABC phenotype is considered; Blumkin et al. [15]; Ferreira et al. [16]; Purnell et al. [17]; Hamilton et al. [18].
R.
Ro
ma
niello
eta
l./Bra
in&
Develo
pm
ent
xx
x(
20
14
)x
xx
–x
xx
5
Please
citeth
isarticle
inp
ressas:
Ro
man
ielloR
etal.
Mu
tation
sin
a-
and
b-tu
bu
linen
cod
ing
genes:
Imp
lication
sin
brain
malfo
rmatio
ns.
Brain
Dev
(2014),h
ttp://d
x.do
i.org/10.1016/j.b
raind
ev.2014.06.002
ha
rr
.

6 R. Romaniello et al. / Brain & Development xxx (2014) xxx–xxx
fusion between the caudate nucleus and putamen, andabsence of the anterior limb of the internal capsule[41]. Moreover, the hippocampi frequently appear mal-rotated with a simplified pattern.
Commissural structures are always involved: the cor-pus callosum is hypoplasic or (partially or completely)agenetic, while the anterior commissure is usually absent.Absence or hypoplasia of the optical nerve tract can alsobe observed. In the posterior fossa, the cerebellum showsvermian and hemispheric hypoplasia and/or dysplasia;the hind-brain is hypoplasic and dysmorphic, sometimeswith a thinned ponto-mesencephalic junction [42].Finally, based on a careful review of the literature wehypothesize that mutations in the tubulin gene superfam-ily cause primary generalized defects in axon guidance;this hypothesis is supported by different findings suchas an altered course of the brainstem tracts, as shownby DTI tractography (personal unpublished data), dys-morphisms of basal ganglia (fusion between the caudatenucleus and putamen with absence of the anterior limb ofthe internal capsule), defects in commissural fiber tracts(anterior commissure and CC abnormalities/agenesis)and fornix, and optic nerve hypoplasia/atrophy.
Fig. 3(A–D) shows the MR features of the extra-cortical brain malformations mainly associated toa-and b-tubulin gene mutations.
4.3. Leukoencephalopathy
Still recently, mutations in the Tubulin b-4A(TUBB4A) gene (ORPHA98805 OMIM 602662) havebeen associated to a novel neuroimaging phenotypecharacterized by hypo myelination with atrophy ofthe basal ganglia and cerebellum (H-ABC) [15–18].
5. Spectrum of the tubulin-related disorders: clinical
aspects
A large spectrum of clinical features is associated withtubulin-related disorders. Motor and intellectual disabil-ity, epilepsy and ocular disorders may be present tovarying degrees. Congenital microcephaly is present inmore than 50% of the patients carrying TUBA1A,TUBB2B and TUBB4A gene mutations and in all thethree patients with TUBB gene mutations. In fact, micro-cephaly is considered by Breuss [14] the distinguishingfeature of TUBB-associated disease. Neither case withTUBB2A gene mutations has microcephaly [13].
Epilepsy has been observed mainly in subjects withTUBA1A, TUBB4A, TUBB2B, TUBB2A (2/2) genemutations and in just over half of the patients withTUBB3 mutations, while it has never been described inTUBB mutated patients. The low association betweenepilepsy and mutations in the latter genes is easy toexplain in TUBB3, while it is difficult to understandfor the TUBB-gene. In fact, TUBB3 is primarily
Please cite this article in press as: Romaniello R et al. Mutations in a- aBrain Dev (2014), http://dx.doi.org/10.1016/j.braindev.2014.06.002
involved in axonal guidance mechanisms and only fewstudies describe its involvement in migration defects[40]. By contrast, TUBB is involved in neurogenic divi-sion and migration processes and it is not clear why itis not associated with epileptic seizures. More patientswith TUBB mutations need to be studied to achieve abetter comprehension of the associated disease and clin-ical patterns.
Cognitive and motor impairments, which are gener-ally severe, are present in almost all patients with muta-tions in a- and b-tubulin genes.
Ocular disorders are especially found in subjects withTUBA8, TUBB3 and TUBB4A gene mutations, in 50%of TUBB2B-mutated patients and only in three caseswith TUBA1A gene mutations. In one subject withTUBB gene mutation, an atypical ocular disorder(retina dysplasia and micro-ophthalmia) has beendescribed. None of the subject with a TUBB2A genemutation presents ocular involvement.
As far as concern TUBB4A, in recent years, a specificphenotype has been described in subjects with dystoniatype 4 (DYT4) characterized by whispering dysphonia,generalized dystonia, ataxic gait (hobby horse) but with-out neuroradiological signs [43,44].
The main clinical aspects associated with a- andb-tubulin gene mutation are summarized in Table 1.
6. Conclusions
A review of the literature and all case reports con-firms the relevance of mutations in tubulin-encodinggenes in determining a large spectrum of brain abnor-malities differently involving the cortex, basal ganglia,hind-brain, cerebellum and commissural structures.These last, especially the anterior commissure defects,seems to be a key feature of tubulin genes mutations.
A wide clinical phenotype is associated with tubulin-related disorders. Motor impairment, intellectual dis-ability and epilepsy are nearly always present to varyingdegrees. By contrast, optical and oculomotor disordersare mainly observed when mutations affect tubulin genesinvolved in axonal guidance mechanisms (TUBB3,TUBA8 and TUBB4A).
7. Funding
This study was partially supported by Italian Minis-try of Health grants to R.B. (RC/01/03/2011) and Insti-tutional funds to MTB and R.B. (5� Mille).
8. Web resources
The URLs for data presented herein are follows:
ORPHANET, http://www.orpha.net/consor/cgi-bin/index.php
nd b-tubulin encoding genes: Implications in brain malformations.

R. Romaniello et al. / Brain & Development xxx (2014) xxx–xxx 7
Online Mendelian Inheritance in Man (OMIM),http://www.ncbi.nlm.nih.gov/omim
References
[1] Barkovich J. Complication begets clarification in classification.Brain 2013;136:368–73.
[2] Sajan SA, Fernandez L, Nieh SE, Rider E, Bukshpun P,Wakahiro M, et al. Both rare and de novo copy number variantsare prevalent in agenesis of the corpus callosum but not incerebellar hypoplasia or polymicrogyria. PLoS Genet 2013;9:e1003823.
[3] Abdollahi MR, Morrison E, Sirey T, Molnar Z, Hayward BE,Carr IM, et al. Mutation of the variant alpha-tubulin TUBA8
results in polymicrogyria with optic nerve hypoplasia. Am J HumGenet 2009;85:737–44.
[4] Jaglin XH, Poirier K, Saillour Y, Buhler E, Tian G, Bahi-BuissonN, et al. Mutations in the beta-tubulin gene TUBB2B result inasymmetrical polymicrogyria. Nat Genet 2009;41:746–52.
[5] Keays DA, Cleak J, Huang GJ, Edwards A, Braun A, TreiberCD, et al. The role of Tuba1a in adult hippocampal neurogenesisand the formation of the dentate gyrus. Dev Neurosci2010;32:268–77.
[6] Kumar RA, Pilz DT, Babatz TD, Cushion TD, Harvey K, TopfM, et al. TUBA1A mutations cause wide spectrum lissencephaly(smooth brain) and suggest that multiple neuronal migrationpathways converge on alpha tubulins. Hum Mol Genet2010;19:2817–27.
[7] Poirier K, Saillour Y, Bahi-Buisson N, Jaglin XH, Fallet-BiancoC, Nabbout R, et al. Mutations in the neuronal b-tubulin subunitTUBB3 result in malformation of cortical development andneuronal migration defects. Hum Mol Genet 2010;19:4462–73.
[8] Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L,He W, et al. Human TUBB3 mutations perturb microtubuledynamics, kinesin interactions, and axon guidance. Cell2010;140:74–87.
[9] Tischfield MA, Cederquist GY, Gupta Jr ML, Engle EC.Phenotypic spectrum of the tubulin-related disorders and func-tional implications of disease-causing mutations. Curr OpinGenet Dev 2011;21:286–94.
[10] Jaglin XH, Chelly J. Tubulin-related cortical dysgeneses: micro-tubule dysfunction underlying neuronal migration defects. TrendsGenet 2009;25:555–66.
[11] Tian G, Jaglin XH, Keays DA, Francis F, Chelly J, Cowan NJ.Disease-associated mutations in TUBA1A result in a spectrum ofdefects in the tubulin folding and heterodimer assembly pathway.Hum Mol Genet 2010;19:3599–613.
[12] Singh KK, Tsai LH. MicroTUB(B3)ules and brain development.Cell 2010;140:30–2.
[13] Cushion TD, Paciorkowski AR, Pilz DT, Mullins JG, Seltzer LE,Marion RW, et al. De novo mutations in the Beta-tubulin geneTUBB2A cause simplified gyral patterning and infantile-onsetepilepsy. Am J Hum Genet 2014;94:634–41.
[14] Breuss M, Heng JI, Poirier K, Tian G, Jaglin XH, Qu Z, et al.Mutations in the b-tubulin gene TUBB5 cause microcephaly withstructural brain abnormalities. Cell Rep 2012;2:1554–62.
[15] Blumkin L, Halevy A, Ben-Ami-Raichman D, Dahari D, HavivA. Expansion of the spectrum of TUBB4A-related disorders: anew phenotype associated with a novel mutation in the TUBB4A
gene. Neurogenetics 2014;15:107–13.[16] Ferreira C, Poretti A, Cohen J, Hamosh A, Naidu S. Novel
TUBB4A mutations and expansion of the neuroimaging pheno-type of hypomyelination with atrophy of the basal ganglia andcerebellum (H-ABC). Am J Med Genet A 2014. http://dx.doi.org/10.1002/ajmg.a.36526 [Apr 4, Epub ahead of print].
Please cite this article in press as: Romaniello R et al. Mutations in a- aBrain Dev (2014), http://dx.doi.org/10.1016/j.braindev.2014.06.002
[17] Purnell SM, Bleyl SB, Bonkowsky JL. Clinical exome sequencingidentifies a novel TUBB4A mutation in a child with statichypomyelinating leukodystrophy. Pediatr Neurol 2014. http://dx.doi.org/10.1016/j.pediatrneurol.2014.01.051 [Feb 10, pii:S0887-8994(14)00086-1, Epub ahead of print].
[18] Hamilton EM, Polder E, Vanderver A, Naidu S, Schiffmann R,Fisher K, et al. Hypomyelination with atrophy of the basalganglia and cerebellum: further delineation of the phenotype andgenotype–phenotype correlation. Brain 2014 [Apr 30, Epub aheadof print].
[19] Tischfield MA, Engle EC. Distinct alpha- and beta-tubulinisotypes are required for the positioning, differentiation andsurvival of neurons: new support for the ‘multi-tubulin’ hypoth-esis. Biosci Rep 2010;30:319–30.
[20] Qu C, Dwyer T, Shao Q, Yang T, Huang H, Liu G. Directbinding of TUBB3 with DCC couples netrin-1 signaling tointracellular microtubule dynamics in axon outgrowth andguidance. J Cell Sci 2013;126:3070–81.
[21] Cushion TD, Dobyns WB, Mullins JG, Stoodley N, Chung SK,Fry AE, et al. Overlapping cortical malformations and mutationsin TUBB2B and TUBA1A. Brain 2013;136:536–48.
[22] Chew S, Balasubramanian R, Chan WM, Kang PB, Andrews C,Webb BD, et al. A novel syndrome caused by the E410K aminoacid substitution in the neuronal b-tubulin isotype 3. Brain2013;136:522–35.
[23] Braun A, Breuss M, Salzer MC, Flint J, Cowan NJ, Keays DA.Tuba8 is expressed at low levels in the developing mouse andhuman brain. Am J Hum Genet 2010;86:819–22.
[24] Poirier K, Keays DA, Francis F, Saillour Y, Bahi N, ManouvrierS, et al. Large spectrum of lissencephaly and pachygyria pheno-types resulting from de novo missense mutations in tubulin alpha1A (TUBA1A). Hum Mutat 2007;28:1055–64.
[25] Bahi-Buisson N, Poirier K, Boddaert N, Saillour Y, Castelnau L,Philip N, et al. Refinement of cortical dysgeneses spectrumassociated with TUBA1A mutations. J Med Genet 2008;45:647–53.
[26] Fallet-Bianco C, Loeuillet L, Poirier K, Loget P, Chapon F,Pasquier L, et al. Neuropathological phenotype of a distinct formof lissencephaly associated with mutations in TUBA1A. Brain2008;131:2304–20.
[27] Morris-Rosendahl DJ, Najm J, Lachmeijer AM, Sztriha L,Martins M, Kuechler A, et al. Refining the phenotype of alpha-1a Tubulin (TUBA1A) mutation in patients with classicallissencephaly. Clin Genet 2008;74:425–33.
[28] Lecourtois M, Poirier K, Friocourt G, Jaglin X, Goldenberg A,Saugier-Veber P, et al. Human lissencephaly with cerebellarhypoplasia due to mutations in TUBA1A: expansion of the foetalneuropathological phenotype. Acta Neuropathol 2010;119:779–89.
[29] Jansen AC, Oostra A, Desprechins B, De Vlaeminck Y, VerhelstH, Regal L, et al. TUBA1A mutations. From isolatedlissenchephaly to familial polymicrogyria. Neurology 2011;76:988–92.
[30] Mokanszki A, Korhegyi I, Szabo N, Bereg E, Gergev G, BaloghE, et al. Lissencephaly and band heterotopia: LIS1,TUBA1A, and DCX mutations in Hungary. J Child Neurol2012;27:1534–40.
[31] Sohal AP, Montgomery T, Mitra D, Ramesh V. TUBA1A
mutation-associated lissencephaly: case report and review of theliterature. Pediatr Neurol 2012;46:127–31.
[32] Hikita N, Hattori H, Kato M, Sakuma S, Morotomi Y, Ishida H,et al. A case of TUBA1A mutation presenting with lissencephalyand Hirschsprung disease. Brain Dev 2014;36:159–62.
[33] Okumura A, Hayashi M, Tsurui H, Yamakawa Y, Abe S, KudoT, et al. Lissencephaly with marked ventricular dilation, agenesisof corpus callosum, and cerebellar hypoplasia caused byTUBA1A mutation. Brain Dev 2013;35:274–9.
nd b-tubulin encoding genes: Implications in brain malformations.

8 R. Romaniello et al. / Brain & Development xxx (2014) xxx–xxx
[34] Poirier K, Saillour Y, Fourniol F, Francis F, Souville I, ValenceS, et al. Expanding the spectrum of TUBA1A-related corticaldysgenesis to Polymicrogyria. Eur J Hum Genet 2013;21:381–5.
[35] Zanni G, Colafati GS, Barresi S, Randisi F, Talamanca LF,Genovese E, et al. Description of a novel TUBA1A mutation inArg-390 associated with asymmetrical polymicrogyria and mid-hindbrain dysgenesis. Eur J Paediatr Neurol 2013;17:361–5.
[36] Cederquist GY, Luchniak A, Tischfield MA, Peeva M, Song Y,Menezes MP, et al. An inherited TUBB2B mutation alters akinesinbinding site and causes polymicrogyria, CFEOM, andaxon dysinnervation. Hum Mol Genet 2012;21:5484–99.
[37] Guerrini R, Mei D, Cordelli DM, Pucatti D, Franzoni E, ParriniE. Symmetric polymicrogyria and pachygyria associated withTUBB2B gene mutations. Eur J Hum Genet 2012;20:995–8.
[38] Romaniello R, Tonelli A, Arrigoni F, Baschirotto C, Triulzi F,Bresolin N, et al. A novel mutation in the b-tubulin gene TUBB2B
associated with complex malformation of cortical developmentand deficits in axonal guidance. Dev Med Child Neurol2012;54:765–9.
[39] Uribe V. The beta-tubulin gene TUBB2B is involved in a largespectrum of neuronal migration disorders. Clin Genet 2010;77:34–5.
Please cite this article in press as: Romaniello R et al. Mutations in a- aBrain Dev (2014), http://dx.doi.org/10.1016/j.braindev.2014.06.002
[40] Saillour Y, Broix L, Bruel-Jungerman E, Lebrun N, Muraca G,Rucci J, et al. Beta tubulin isoforms are not interchangeable forrescuing impaired radial migration due to Tubb3 knockdown.Hum Mol Genet 2014;23:1516–26.
[41] Amrom D, Tanyalc�in I, Verhelst H, Deconinck N, Brouhard G,Decarie JC, et al. Polymicrogyria with dysmorphic basal ganglia?Think tubulin! Clin Genet 2013. http://dx.doi.org/10.1111/cge.12141 [Mar 15, Epub ahead of print].
[42] Romaniello R, Arrigoni F, Cavallini A, Tenderini E, BaschirottoC, Triulzi F, et al. Brain malformations and mutations in a and btubulin genes: a review of the literature and description of twonew cases. Dev Med Child Neurol 2014;56:354–60.
[43] Hersheson J, Mencacci NE, Davis M, MacDonald N, TrabzuniD, Ryten M, et al. Mutations in the autoregulatory domainof b-tubulin 4a cause hereditary dystonia. Ann Neurol 2013;73:546–53.
[44] Lohmann K, Wilcox RA, Winkler S, Ramirez A, Rakovic A,Park JS, et al. Whispering dysphonia (DYT4 dystonia) iscaused by a mutation in the TUBB4 gene. Ann Neurol 2013;73:537.
nd b-tubulin encoding genes: Implications in brain malformations.














![Journal of Controlled Release · Mertansine (DM1) is a powerful tubulin polymerization inhibitor that can effectively treat various malignancies including breast cancer, melanoma,multiplemyelomaandlungcancer[1,2].TherecentFDAap-](https://static.fdocument.org/doc/165x107/6022d870e69dd92acd3aabf0/journal-of-controlled-mertansine-dm1-is-a-powerful-tubulin-polymerization-inhibitor.jpg)



