IN SITU CLICK CHEMISTRY
Transcript of IN SITU CLICK CHEMISTRY

COVER STORY
PERFECT FIT Model of acetylcholinesterase inhibitor.
IN SITU CLICK CHEMISTRY
Templating strategy offers potential route to new drugs and
other functional compounds STU BORMAN, C&EN WASHINGTON
Ν THE "CLICK CHEMISTRY" STRATEGY DEVELOPED RECENT-
ly at Scripps Research Institute, reactive molecular building blocks are designed to "click" together selectively and cova-lently The Scripps researchers are now extending the idea by using protein binding sites, supramolecular complexes, or
ftinctionalized surfaces as reaction vessels to direct the in situ formation of potentially functional click chemistry products. The products might be biological inhibitors, molecular-electronics components, sensor probes, nonlinear optical materials, light-harvesting compounds, or compounds with any number of other useful properties.
In what might be considered either a lucky break or a harbinger of the technique's power, the scientists used the procedure successfully on its first tryout to identify an inhibitor of the disease-associated enzyme acetylcholinesterase. The inhibitor binds at femtomolar levels—an ex
traordinarily strong measure of binding affinity [Angew. Chem., in press}.
Click chemistry is the use of chemical building blocks with "built-in high-energy content to drive a spontaneous and irreversible linkage reaction with appropriate complementary sites in other blocks," explains Scripps chemistry professor and Nobel Laureate K. Barry Sharpless, who conceived the strategy [Angew. Chem. Int. Ed., 40, 2004 (2001)]. The expanded strate
gy—in situ click chemistry—is the use of chemical and biological receptor structures as templates to guide the formation of click chemistry products.
There are powerful and plentiful precedents for such a templating concept, and even for the reaction Sharpless and coworkers are employing in their initial studies. But nobody has put those elements together into an overall strategy for drug and materials discovery in just this way before.
In recent years, Sharpless says, there has been "an enormous emphasis on the structure-guided synthesis of molecules with desired properties. Unfortunately, successes have been rare. For one thing, our understanding of the systems involved is often not good enough, and the number of molecules that could be considered is too vast."
In medicinal chemistry for example, the traditional approach is to lock in the structures of drug candidates ahead of time— "before they are tested against a biological
H T T P : / / P U B S . A C S . O R G / C E N C & E N / F E B R U A R Y 1 1 , 2 0 0 2 2 9

COVER STORY
target," Sharpless notes. "This traditional strategy often seems only marginally better than a shot in the dark. Wouldn't it be more productive if the biological system could somehow be directly engaged in the synthetic sequence leading to the drug candidate?"
ANY SEARCH PROCESS "that actually de pends on the biological target structure serving as the catalytic mold—for creating new molecules that interfere with or change its normal function—should enable a quantum leap in the effectiveness of drug discovery endeavors," he adds. 'When the appropriate reactive modules are provided, a form-fitting small-molecule inhibitor will be erected right in the heart of the enzyme's catalytic machinery." For biological and nonbiological applications alike, "this promises to be a generic way of engaging chemical reactivity to search for and create useful functions."
Asked to comment on the in situ click chemistry strategy, bioorganic chemist Ivan Hue of the European Institute of Chemistry & Biology, Bordeaux, France, tells C&EN that the initial results by Sharpless and coworkers, in which a femtomolar in- = hibitor was found, "are tru- Ë ly remarkable in terms of ï efficiency"Althoughthein < situ part of the procedure £ is "conceptually not new," £ the findings demonstrate 5 that the strategy of enzyme § templating "can be more © successful than was ever | shown before." °
David C. Rees, head of | medicinal chemistry at As- J traZeneca, Môlndal, Swe- ο den, comments that "the | idea of using an enzyme's £ binding site to fish out two £ small organic reactants and î then force them to react with each other stereoselectively is really very elegant." Sharpless and coworkers have "applied the idea to synthesize a novel and very high-affinity inhibitor for a much-studied enzyme. It allows one to speculate that it might become possible to use proteins or other biopolymers as 'super reagents' or catalysts for synthesizing compounds with useful properties."
Ideally, click chemistry reagents are
NESTLED Model of inhibitor in the active site of acetylcholinesterase, similar to one formed experimentally using in situ click chemistry.
spring-loaded to combine pretty much only with each other. At the same time, for biological applications, "these spring-loaded sites must be virtually inert toward the chemicals found in living systems," Sharpless says. This "should enable one to use them for self-assembly reactions right in the middle of functioning biosystems."
IN SITU G ROUP Sharpless (top left), coworkers (from left) Radie, Grynszpan, Green, and Lewis, and collaborator Carlier (bottom left).
In addition, "the blocks will often house structural features well known for their polar or hydrophobic binding affinities with biological structural motifs."
Building blocks must also be designed to combine only when they are positioned very close to one another. This makes it possible for the active site of a biomole-cule to act as a template for the combination process. Only if the building blocks'
polar or hydrophobic features bind to specific active-site structures do the blocks line up next to each other, permitting them to click together like Lego pieces.
For now, the reactive building blocks on which Sharpless and coworkers are focusing have azide and acetylene groups that combine readily with each other—when held in close proximity— to form triazoles. This type of cycloaddition reaction was one of many first studied in detail by chemistry professor emeritus Rolf Huisgen of the University of Munich, in Germany If the polar or hydrophobic structural groups of one azide-containing and one acetylene-containing compound find adjacent binding sites on a templating enzyme, the compounds react with each other to form a triazole product that is likely to inhibit the enzyme.
When the positioning is right, the cycloadditions "are strongly driven, almost inevitable transformations, depending on little else except the effective molalities of the reactants," Sharpless says.
SCRIPPS ASSOCIATE professor of chemistry M. G. Finn, one of Sharpless' collabo
rators, notes that a practical benefit of the strategy is that detecting a hit (an inhibitor of a protein target) "relies not upon characterizing the target protein's function in the presence of the candidate, but rather on detecting the production of a new small molecule. The analytical challenges to screening large numbers of combinations are thereby changed, sometimes for the better."
However, using an enzyme or enzymelike molecule to template a bimole-cular reaction is not a novel concept and in fact has a
venerable history "Others have developed the idea of asking a target to 'template' the construction of form-fitting small-molecule inhibitors," Finn notes. Perhaps the first, he says, were lecturers James F. A. Chase and Philip K. Tubbs of the department of biochemistry at Cambridge University, who reported in 1969 on the self-catalyzed inactivation of carnitine acetyltransferase. "The difference in our
"Wouldn't it be more productive if the biological system could be directly engaged in the synthetic sequence?" 3 0 C & E N / F E B R U A R Y 1 1 , 2 0 0 2 H T T P : / / P U B S . A C S . O R G / C E N

approach is not the philosophy of template assembly, but rather the chemistry that we use to accomplish ligations of blocks in the target," Finn says.
An important chemical precedent for in situ click chemistry was a series of classic experiments in the 1980s by chemistry professor William L. Mock of the University of Illinois, Chicago, and coworkers on cucurbituril—a nonadeca-cyclic cage structure that acts as a catalyst. The major differences are that Mock and coworkers did not use an enzyme and did not approach templating as a way to create functional compounds (such as enzyme inhibitors).
Mock's group used cucurbituril as a templating agent to guide the regiospecific formation of triazole products from the union of azide and alkyne substrates. In these experiments, only single-isomer triazole products were formed, whereas nontem-plated cycloadditions of the same components yield approximately 1:1 mixtures of isomeric syn- and anti-triazoles.
Mock and coworkers estimated that "cucurbituril achieved a rate acceleration of 100,000 over the background thermal process," Sharpless says. "They also encountered the same binding-constant measurement problem we did. Their in situ tem-plated triazole, although noncovalent and rather small, was barely able to escape from the cozy channel that fit it like a glove." Such tight binding makes it difficult to carry out kinetic studies and to isolate enough templated product for identification.
SHARPLESS SAYS he "cannot get over the nearly perfect match between Mock's cucurbituril experiments and our acetylcholinesterase experiments done 20 years later. If cucurbituril had been a small enzyme, then the two systems would be indistinguishable." However, the implications of templating for drug discovery are more apparent today than they were at the time Mock and coworkers did their classic experiments.
Researchers active more recently in templating by enzymes and enzymelike complexes include Darryl C. Rideout of Structural Bioinformatics, San Diego; chemistry professor and Nobel Prize-winner Jean-Marie Lehn and coworkers at University Louis Pasteur, Strasbourg, France; the group of chemistry professor Stephen J. Benkovic at Pennsylvania State University, University Park; Hue's team in Bordeaux; chemistry professor K. C. Nicolaou and coworkers at Scripps and the University of California, San Diego; and director of chemistry Alexey V Eliseev's group at
Therascope AG, Heidelberg, Germany Their contributions include the
following: • Rideout reported in 1986 on how cy-
totoxins and drugs could self-assemble in vivo via the formation of hydrazones from aldehydes and hydrazine derivatives. The reagents reacted with one another inside living cells irreversibly but did not react "at a significant rate with biomolecules at physiological concentrations," Rideout says.
• Lehn's group developed the concept
of target-driven dynamic combinatorial chemistry, in which building blocks with properties suitable for forming a supramol-ecular entity are selected from a combinatorial library and assembled in the presence of a target.
• And Eliseev and coworkers recently used neuraminidase as a template for the selective synthesis of inhibitors from highly diverse dynamic combinatorial libraries [Proc. Natl. Acad. Sa. USA, in press}. They found that aldehyde and amine building blocks combined by reductive animation
^accelrys
ÎFlCAChe*
pDINGEi^ I
For the BEST Stereo Viewing...
Look No Further.
^^^^EYES^3
When you need to analyze complex molecular structures, you need the best visualization tools. Used by industry leaders, StereoGraphics' CrystalEyes3 and Monitor ZScreen 2000/are the professional's choice.
"Viewing the models in Stereo3Dm
really enables the audience to picture what is going on and to view it better than in a flat, 2D environment By using CrystalEyes, it is easier to explain how these inhibitors interact with HIV protease and the molecular mechanism of the protease inhibitors.'1 Eric Furfine
GlaxoSmithKline
Whether you are looking for active shutter eyewear or a passive polarizing system, StereoGraphics Corporation has the best solution for your stereo viewing needs.
STEREOGRAPHICS 9
For More Information: Call Toll Free: 866-455-1490 Visit: www.stereographics.com/cen/ Email: [email protected]
GIS/Mapping · Molecular Modelling · CAD · Industrial VR * Medical Imaging · Simulation
H T T P : / / P U B S . A C S . O R G / C E N C&EN / FEBRUARY
CEN021102
2002 31

COVER STORY
^
R N ^ " Ν * *
syn anti
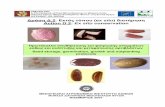
TEMPLATED REACTION Generic azide-acetylene reaction in its nontemplated form (inset, top left) produces equal amounts of syn- and anti-isomeric products. But when acetylcholinesterase (blue) was used as a template for the reaction of acetylene (left) and azide (right) building blocks, the syn addition product (structure at upper right and spacefilling model in enzyme active site) from the two highlighted azide and acetylene building blocks was the only compound formed. The product turns out to be the strongest noncovalent inhibitor of acetylcholinesterase yet identified.
to form inhibitors only in the presence of the enzyme.
However, Sharpless notes that many previous reports of templated reactions taking place within enzymes relied upon the reaction of a thiol with a halocarbonyl compound. One drawback of this approach, he says, is that halocarbonyl compounds react readily with enzyme nucle-ophilic sites, which can alter the enzymes and even block the very binding sites one is trying to probe.
The reaction of azides and acetylenes to form triazoles sidesteps this problem. According to Sharpless, "The building blocks are effectively orthogonal, within limits, to the typical reactive groups and conditions encountered in enzymes and most other biological systems"—that is, they don't tend to cross-react with bio-
molecules. Hue comments that the Sharpless group's use of the azide-acetylene reaction represents "true progress" because of its high selectivity
Some azide- and acetylene-based building blocks could theoretically react with each other to a limited extent when they're free in solution, before they even encounter an enzyme template. But by holding them near each other, the enzyme speeds up the process greatly
"UNLESS THE PIECES are held close together, the reaction is kinetically slow," Finn explains. "It has a reasonably high activation barrier but is thermodynamically very favorable. Most importantly, a significant portion of the activation barrier is entropie—the pieces have to approach each other in precisely the right orienta
tion. Holding the pieces close together with something else, like an enzyme binding pocket, helps pay this entropie cost and thereby accelerates the process."
The selectivity of the reaction, Finn points out, is reminiscent of that of target-accelerated combinatorial synthesis, an olefin metathesis strategy developed by Nicolaou and coworkers in which the starting materials are similarly unreactive with biocompounds. Two years ago, Nicolaou's group used such reactions to construct highly potent vancomycin dimers.
Sharpless says his group's choice of the azide-acetylene reaction—"the most versatile and reliable of all the Huisgen [3+2} cycloaddition processes—was, in retrospect, probably inevitable. Not only is it highly exergonic, but it is a pure fusion process needing only concerted reorgani-
3 2 C & E N / F E B R U A R Y 1 1 , 2 0 0 2 H T T P : / / P U B S . A C S . O R G / C E N

zation of several π bonds into two new σ bonds." Reaction yields are as high as 99% or more, although he concedes that reaction rates can be slow.
In their initial in situ click chemistry study Sharpless and collaborators used the active site of electric eel acetylcholinesterase to template the formation of their fem-tomolar inhibitor. Although the nontem-plated azide-acetylene reaction forms 50-50 mixtures of syn- and anti-product isomers, the enzyme-templated reaction yielded only the syn form. This syn product was, in fact, the only triazole that formed out of 98 possible reaction products from all combinations of the azide and acetylene building blocks used.
"This is the most potent noncovalent inhibitor ever found for the acetylcholinesterase system, by at least two orders of magnitude," Sharpless tells C&EN—"not bad for our first effort to create an enzyme inhibitor in situ. Acetylcholinesterase is a crucial enzyme in the mammalian nervous system and a current target for drugs to alleviate Alzheimer's disease."
Sharpless' collaborators on the acetylcholinesterase study were graduate student
Warren G. Lewis, research associate Luke G. Green, assistant professor of chemistry Flavio Grynszpan, and Finn at Scripps; assistant project scientist Zoran Radie and professor Palmer W. Taylor at the department of pharmacology of the UC San Diego School of Medicine; and associate professor of chemistry Paul R. Carlier of Virginia Polytechnic Institute & State University. Sharpless also credits chemistry professor Daniel W Armstrong and graduate student Clifford R. Mitchell of Iowa State University, Ames, for chromatographic assistance.
THE REACTION RATE for the cycloaddition system used in the acetylcholinesterase study was extremely slow—"so slow, in fact, that we nearly failed to detect the tem-plated triazole," Sharpless says. However, "the rate of the cycloaddition process is easily 'tuned' and can be increased several hundredfold," he notes. And because of the high yield of click reactions, he believes compounds discovered this way could be produced inexpensively and thus evaluated relatively easily "We could make, within a week, 100 g of any compound from an
initial screening library that exhibits interesting activity," he says.
AstraZeneca's Rees comments that ' what is exciting from a drug discovery perspective is the prospect that in situ click chemistry could be extended to identify other small-molecule ligands or modulators for proteins or biopolymers, even when the structure or function of the protein is unknown. This is a big challenge and often a bottleneck for medicinal chemists in the pharmaceutical industry today, especially in view of the number of protein targets that have arisen from unraveling the human genome."
High-throughput biological screening of large chemical libraries can be used successfully to identify such hits, Rees says, "but there is a drive for alternative and complementary techniques, and this is just one of the areas in which in situ click chemistry will attract attention."
However, he adds, "it is too early to be sure how generally applicable in situ click chemistry will be. Acetylcholinesterase may prove to be particularly well suited to this approach due to its lipophilic cleft and well-defined binding pockets."
RAPID XRD COMBINATORIAL SCREENING D8 DISCOVER with GADDS
ess·*- wl,,z „„d opera»0"''
batch scrip* •«..*
techniques
BRUKER ADVANCED X-RAY SOLUTIONS USA and Canada: Tel. (+1) 608/276-3000 · Fax (+1) 608/276-3006 · http://www.bruker-axs.com · E-mail: [email protected] Worldwide: Tel. (+49) 721/595-2888 · Fax (+49) 721/595-4587 · http://www.bruker-axs.de · E-mail: [email protected]
IHKUKER axs

... was behind the concept that led to the development of the compact disc?
... played a significant role in the development of the Xerox copier?
... recommended the bar code symbol found on millions of consumer products?
... developed the protective coating on golf balls that prevents them from cracking?
If you said congratulations. You are absolutely correct.
At Battelle, we're in the business of innovation. We serve industry and government by developing the technology behind some of the most successful products in the world. With a staff of 7,500 scientists, engineers, and support specialists, we work on fascinating projects that result in between 50-100 patented inventions each year.
To learn more about our remarkable organization please visit our website at www.battelle.org. EOE M/F/D/V.
Battelle
T_KFM* Scientific, %J Π ^ . m ^J Ι ψ JL_ Instruments for^cietice from KSaer,
Phone: (800) 827-4W9 n C . (314)863-5536
•ufc JKFA191 l(«JKEM.COM
Instruments for Tomorrow's Discoveries Temperature Controllers
prrecision _
'Simplicity L *
Safety
\
W
ί'ίχλ
Gemini dual channel controller
NEW!! Solvent Evaporator
* Guaranteed 0.1° C regulation of:
• Λη\ volume • An> heater
* No overshoot of entered temperature
* Ramp-to-Setpoint feature ( 100 steps)
* Computer control • Control reactions • Log data
N E W ! Oil bath control!·
Parallel Synthesis Reactors
24 Position KEM-Prep Reactor * 25 & 50ml glass reactors * Temperature range: -100 to 130°C * Inert ar^on atmosphere * Teflon septum cover * Heat, Cool. Reflux reactions
y
Evaporates: Acetone * DMSO
D M F * Water * TFA
Concentrators for: Titer plates * Gilson racks
Custom designs
Accessories Include: * Ν : gas heater * Programmable
vacuum controller
<$5K Complete
'è Multi-Vial KEM-Lab Reactor
* Heated and Cooled reactors for 2, 4, 8, 16, 20ml vials & titer plates
* Built-in heater
* Built-in block temperature ramping
In the nonbiological area, Sharpless, Finn, and coworkers are collaborating with groups led by chemistry professors Paul S. Weiss and Raymond L. Funk of Penn State and James M. Tour of Rice University in an effort to develop surfaces with precise chemical patterns at the nanometer scale. They hope to collaborate with chemistry professor Timothy M. Swager and coworkers at Massachusetts Institute of Technology on click chemistry-based sensors. 'And there are endless possibilities in surface science, chromatography, and other areas," Sharpless says.
THE APPROACH USED in these nonbiological studies "is basically the same as for the enzyme inhibitors," Sharpless says, "except that the molecular-scale reaction guide might now be—instead of an enzyme active site—a surface that templates azide-and acetylene-decorated pieces together if they are recruited to adjacent sites." Alternatively, the synthesis might now involve "a unique selection of polyvalent azide-acetylene blocks that generate an oligomeric structure with a defined repeating motif, encoded by complementary recognition elements in the assembling blocks. The beauty of these nonbiological projects is that a discovery can lead much more directly to real uses."
What pure chemistry offers, he says, "is constant advances in our ability to improve and understand chemical reactivity, which in turn makes it easier to make existing, useful chemical entities and to discover new ones faster. This brings me back to a quote" from former California Institute of Technology chemistry professor George S. Hammond "about 'properties,' not compounds, being the goal of synthesis. There are too many compounds for any one of them to be sacred, but the reactivity that connects them to each other is."
Sharpless conceived of click chemistry as a fast, high-yield form of organic synthesis several years ago. But he says he only realized the implications of using it for in situ synthesis in biomolecular active sites in April 2000, when it "suddenly jumped out at me that Huisgen's triazole synthesis was in a class by itself. It sure has taken us a long time to prove that the in situ idea actually works, but when we finally discovered Mock's precedent it became instantly clear that our present enzyme-based version had to work, too. I have always been fascinated by the way in which simple and yet very important connections remain invisible to us until their time to be seen approaches and some idiosyncrasy in our vision brings them out." •
3 4 C & E N / F E B R U A R Y 2 0 0 2 H T T P : / / P U B S . A C S . O R G / C E N