EXPRESSION OF ESTROGEN RECEPTOR α AND PROGESTERONE RECEPTOR IN CHILDREN WITH UNDESCENDED TESTICLE...
Transcript of EXPRESSION OF ESTROGEN RECEPTOR α AND PROGESTERONE RECEPTOR IN CHILDREN WITH UNDESCENDED TESTICLE...
EXPRESSION OF ESTROGEN RECEPTOR � AND PROGESTERONERECEPTOR IN CHILDREN WITH UNDESCENDED TESTICLE
PREVIOUSLY TREATED WITH HUMAN CHORIONIC GONADOTROPIN
PRZEMYSLAW PRZEWRATIL, DARIUS A. PADUCH,* JOZEF KOBOS AND JERZY NIEDZIELSKIFrom the Departments of Pediatric Surgery and Oncology (PP, JN), and Pathology (JK), Medical University of Lodz, Lodz, Poland, andCenter for Biomedical Research, Population Council and Department of Urology, Weill Medical College, Cornell University, New York,
New York (DAP)
ABSTRACT
Purpose: The aim of this study was to investigate expression of estrogen receptor � (ER�) andprogesterone receptor (PR) in paratesticular tissues obtained from boys with undescended testes.Materials and Methods: A total of 65 boys with unilateral cryptorchidism and failed human
chorionic gonadotropion treatment underwent orchiopexy. A small sample of gubernaculum,cremasteric muscle and processus vaginalis was obtained. A total of 57 boys who underwentinguinal hernia repair served as the control group. All boys in the control group had testes in thescrotum. The expression of estrogen receptor � and progesterone receptor was measured bycounting the number of ER� or PR positive cells detected by immunohistochemical analysis.
Results: ER� and PR density was higher in cremasteric muscle and processus vaginalisobtained from boys with undescended testes than in the control group. Density of progesteronereceptor in the examined groups was lower than the density of estrogen receptor.Conclusions: ER� and PR are expressed in paratesticular tissues important for normal testic-
ular descent. ER� was over expressed in cremasteric muscle and processus vaginalis in boys withundescended testes previously treated with human chorionic gonadotropin.
KEY WORDS: cryptorchidism; receptors, estrogen; receptors, progesterone; testis
The proper position of the testes is at the bottom of thescrotum. Cryptorchidism, found in 2% to 4% of newborns, isa developmental disorder in which descent of the testis intothe scrotum is disrupted. The incidence of cryptorchidismdecreases to 0.5% to 1% by age 1 year due to spontaneousdescent.1 Cryptorchidism may lead to impaired fertility andhigher risk (3 to 8 times) of a testicular tumor, and althoughorchiopexy does not prevent the neoplasm, surgical correc-tion allows for easier testicular examination.Regulation of testicular descent is poorly understood. The
testis develops in the abdominal cavity and descends cau-dally to achieve its scrotal position. Transabdominal migra-tion and transinguinal descent require normal anatomicalconfiguration and adequate hormonal stimulation.The association of cryptorchidism with the prune belly
syndrome suggests that adequate intra-abdominal pressureand normal anatomy are paramount to testicular descent. Onthe other hand, cryptorchidism seen in the androgen insen-sitivity syndrome emphasizes the role of hormones in testic-ular descent. One can assume a multifactorial etiology ofcryptorchidism with close interplay of anatomical, hormonaland potentially environmental factors.2
In experimental models androgens, calcitonin gene-relatedpeptide, epidermal growth factor and anti-mullerian hor-mone, among many other factors, are involved in testiculardescent. However, the importance and role of each factor inhumans is not clear. Testosterone has traditionally beenconsidered a pure male hormone and estrogen a pure femalehormone. However, we now know that a small amount ofestrogen is critical for normal development of bones, muscle,
brain and testis in men. Testosterone is converted by aro-matase CYP19 to estradiol in many tissues obtained fromhealthy men. Lack of estrogen in men can result in kypho-scoliosis and osteopenia. Estrogen excess can lead to infertil-ity and hypogonadism.3
Testosterone is needed for involution of the cranial suspen-sory ligament and transinguinal descent.4 Husmann andMcPhaul suggested the presence of androgen receptors in thegubernaculum in the early postnatal period only (up to age 2weeks).5 Androgen receptors were found in the cremastericmuscle of boys with cryptorchidism, while they were absentin the gubernaculum. Hence, it is possible that factors otherthan androgens have a dominant role in the late phase oftesticular descent.6
Insulin-like hormone 3 (insl-3 or relaxin) is involved in thefunction of the gubernaculum.6 Insl-3 is under estrogen con-trol, as evidenced by experimentally induced cryptorchidismin animals exposed to diethylstilbestrol.7 Since screening formutations in insl-3 gene showed a low incidence of 1.3% in145 patients with a history of cryptorchidism, it is possiblethat estrogen may affect insl-3 expression, and, thus, mayhave a role in the regulation of testicular descent.8 However,the mechanism of estrogen control of this process is stillunknown. It is also unknown at this point whether maternalexposure to endocrine disruptors with estrogen-like actioncan contribute to the development of cryptorchidism. The roleof another product of steroidogenesis, progesterone, in thedescent process remains completely unknown. Exposure toestrogen-like hormone disruptors found in food and the en-vironment has been implicated to have a role in cryptorchid-ism, hypospadiasis and the development of testicular can-cer.9
The experimental data linking cryptorchidism to excessiveestrogen exposure, and our interest in the role of estrogen-like pollutants on human sexual development prompted us toinvestigate the potential role of estrogen and progesterone on
Accepted for publication April 8, 2004.Study received internal review board approval.* Correspondence: Department of Urology, New York Presbyterian
Hospital-Weill Cornell Medical Center, 525 East 68th St., New York,New York 10021 (telephone: 212-327-8740; FAX: 212-327-7678; e-mail:[email protected]).
0022-5347/04/1723-1112/0 Vol. 172, 1112–1116, September 2004THE JOURNAL OF UROLOGY® Printed in U.S.A.Copyright © 2004 by AMERICAN UROLOGICAL ASSOCIATION DOI: 10.1097/01.ju.0000134918.61279.98
1112
testicular descent. In this study we evaluate the hypothesisof differences in expression of estrogen and progesteronereceptors in paratesticular tissues of boys with and withoutcryptorchidism. The aims of this study were to investigatethe presence of estrogen receptor � (ER�) and progesteronereceptor (PR) in paratesticular tissues obtained from boyswith unilateral cryptorchidism undergoing orchiopexy; totest the hypothesis that boys with cryptorchidism will havehigher expression of estrogen and progesterone receptors inparatesticular tissues than those with descended testicles;and to evaluate the relationships between the position of thetestis before surgery and the receptor expression pattern insampled tissues.
MATERIALS AND METHODS
A total of 189 boys 2 to 12 years old with unilateral cryp-torchidism underwent orchiopexy between 1998 and 1999. Ofthis group 65 boys agreed to participate and were included inthe current study. All boys failed to respond to hormonaltreatment with human chorionic gonadotropin (HCG) givenintramuscularly. Hormonal therapy was finished at least 6months before surgery. A total of 142 specimens, obtainedduring surgery, were stored and analyzed using the samemethod. A total of 55 samples of cremasteric muscle, 33samples of processus vaginalis and 54 samples of gubernac-ulum were processed. Patient age and position of the testisbefore surgery were recorded. Position of the testis at surgerywas classified as being in the abdominal cavity, near theinternal inguinal ring, in the inguinal canal, near the exter-nal inguinal ring or ectopic. Ectopy was diagnosed duringsurgery as testis outside the scrotum but with spermatic cordpassing through the external ring.The control group consisted of 57 boys 2 to 10 years old who
underwent inguinal hernia repair with testes in the scrotum.In controls only cremasteric muscle (45 specimens) and pro-cessus vaginalis (52 specimens) were obtained, since the gu-bernaculum would be technically difficult to obtain. Thenumber of samples in each group is different from the num-ber of patients, because only tissues of suitable quality andquantity were processed. Paraffin sections were used forroutine hematoxylin and eosin (H & E) staining and immu-nohistochemical analysis with alkaline phosphatase detect-ing system (streptavidin-biotin K0678, Dako Corp., Carpin-teria, California).After tissues were fixed with neutral buffered formalin
they were embedded in small paraffin blocks. Four �m sec-tioned tissues were collected on clean glass slides and dehy-drated at 56.7C for 24 hours. Next, they were deparaffinizedin xylene and ethanol series. To expose the antigens, theslides were immersed in 0.01 M citric buffer (pH 6) andheated in a microwave oven to 90C. The slides were thenwashed with 0.05 M tris hydrochloric acid (HCl) (pH 7.4)buffer twice (5 minutes).Primary antibody was diluted with tris buffered saline
(TBS) using the ratio recommended by Dako Corp. (ER� 1:50,PR 1:10). The specimen was covered with diluted antibodyand incubated at room temperature for 15 minutes. Next,incubation sections were rinsed in 0.05 M tris-HCl buffer andplaced in a fresh buffer bath.Biotinylated anti-mouse antibodies in phosphate buffered
saline containing carrier protein and 15 mM sodium amidewere applied to the wiped slides and the slides were incu-bated for 15 minutes, followed by a 5-minute wash with TBS.The slides were wiped gently and streptavidin conjugated toalkaline phosphatase in phosphate buffered saline was ap-plied for 15 minutes at room temperature.After wash with TBS chromogen solution was applied
(naphthol phosphate in tris-HCl buffer) for 15 minutes. Theslides were washed with distilled water for 2 minutes andtissues were counterstained with hematoxylin, rinsed with
distilled water and submerged 10 times into a wash bathfilled with 37 mM ammoniac water, followed by a final washin distilled water for 2 minutes. The slides were mountedwith Glycergel mounting medium (C0563, Dako Corp.).Agents used for immunohistochemical study included
monoclonal mouse anti-human estrogen receptor �, clone1D5 (catalog No. M 7047, lot 057, antigen—recombinant hu-man estrogen receptor protein, Dako Corp.) and monoclonalmouse anti-human progesterone receptor, clone 1A6 (catalogNo. 3529, lot 077, antigen—Synthetic peptide, Dako Corp.).Breast cancer specimen with strong immunohistochemicalreaction for estrogen and progesterone receptors was used asa positive control. Negative control (breast cancer specimen)slides lacked primary antibody against receptors.The following semiquantitative score was used to measure
density of receptor expression per cell—no expression (0),single (1 to 4), several (5 to 9), 10 to 20 and more than 20 cellsper high power field. A digital morphometric system wasused to assess the median number of receptor positive cellsfrom 5 fields in each specimen. Photomicrographs were takenwith a Microphot-FXA (Nikon, Melville, New York) and pro-cessed with a MultiScanBase version 8.09 (Computer Scan-ning Systems, Ltd., Warsaw, Poland) image analysis system.Stained slides were coded and double blinded to objectifyhistopathological assessment. Chi-square, Spearman andKolmogorov-Smirnov tests were used for statistical analysis.Informed consent was obtained from the parents before ob-taining tissue samples.
RESULTS
There was no statistically significant difference in age dis-tribution.Cryptorchidism group. Estrogen and progesterone recep-
tors were detected respectively in 36 (65%) and 19 (35%) of 55cremasteric muscle specimens (see table and figs. 1 to 3). Ofthe 33 processus vaginalis specimens 22 (67%) had positiveimmunostaining for ER� and 11 (33%) for PR. Similar resultswere noted in 54 gubernaculum specimens—36 specimens(67%) had a positive ER� reaction and 17 (32%) expressedPR. Density of receptor expression per field differed amongthe specimens from single to several cells.Control group. Of 45 cremasteric muscle specimens expres-
sion of ER� was found in 7 (16%) and PR in only 1 (2%). ER�was found in 11 (29%) and PR in 1 (3%) of 38 samples ofprocessus vaginalis. However, receptor immunoreactivitywas seen in single cells only.Expression of ER� in tissues obtained from patients with
cryptorchidism was significantly higher than in the controlgroup. This was true for cremasteric muscle (chi-square23.10, p �0.0001) and processus vaginalis (chi-square 17.61,p �0.0001). Similarly, expression of PR in the cryptorchidgroup was higher in cremasteric muscle (chi-square 14.20,p �0.0003) and in processus vaginalis (chi-square13.94, p �0.0003) compared to the control group. Proges-terone receptor was expressed in smaller quantity thanestrogen receptor in all samples (fig. 4).Presence of progesterone receptor seems to have a better
predictive value than presence of estrogen receptor. Proges-terone receptor was found in 2% to 3% of specimens from thecontrol group but estrogen receptor was found in 16% to 29%of specimens from the control group. Calculated ratio of pos-itive specimens in cryptorchidism and control groups was16.7 for progesterone receptor and 3.7 for estrogen receptor.There was no statistically significant correlation between
patient age and density of estrogen or progesterone receptorexpression. Nonparametric tests did not meet p �0.05 signif-icance value to prove the potential relationship between theposition of the testis and receptor density. It can be attrib-uted to 10-fold differences between numbers of cases in eachposition cell.
EXPRESSION OF ESTROGEN AND PROGESTERONE RECEPTORS IN UNDESCENDED TESTIS 1113
DISCUSSION
An increasing incidence of male reproductive tract abnor-malities (cryptorchidism, hypospadias), testicular cancer anddeterioration of sperm quality has been demonstrated inseveral studies, especially in industrial countries. This find-ing may be linked to potential exposure to industrial andagricultural estrogens or estrogen-like substances.9 Food,
prescription drugs, self-medication with herbal remedies, ag-ricultural and industrial exposure, household products andenvironmental pollution with endocrine disruptors are con-sidered potential sources of such agents. Endocrine disrup-tors and estrogen exposure can modify normal testiculardescent by central and peripheral mechanisms. Estrogensmay directly inhibit release of gonadotropin-releasing hor-mone, luteinizing hormone (LH) and follicle-stimulating hor-mone from the hypothalamus and pituitary. Direct binding ofestrogens to cytosol estrogen receptor in the effector cellsduring organogenesis and testicular descent may modulateexpression of LH and follicle-stimulating hormone recep-tors.10 Since estrogen response elements are found in manytranscription complexes, it is possible that excessive estrogenexposure can disrupt normal male development and func-tion.11 It has also been noticed that, although weak, environ-mentally present estrogens can have a cumulative, time de-pendent mechanism of action.12ER� knockout mice deprived of the proper estrogen action
were infertile and had retractile testicles.13 Damber et alfound increased intratesticular concentration of estradiol-17� and estrogen receptor expression in rats with surgicallyproduced cryptorchidism.14 These facts can be taken as proofof the important role of estrogen receptors in the develop-ment and function of the male genitourinary tract but theexact molecular mechanism is not clear.
Cumulative results of immunohistochemical reaction for ER� and PR in tissues obtained from boys with undescended testicle vs controls
Immunostain Neg†Stain Intensity* Total
SpecimensSingle Cells Several Cells 10–20 Cells More Than 20 Cells‡
Cremasteric muscle:Cryptorchidism ER� 19 22 7 4 3 55
PR 36 10 2 6 1 55Control ER� 38 6 1 — — 45
PR 44 1 — — — 45Processus vaginalis:Cryptorchidism ER� 11 16 4 2 — 33
PR 22 5 4 1 1 33Control ER� 41 9 2 — — 52
PR 51 1 — — — 52Gubernaculum:Cryptorchidism ER� 18 23 7 4 2 54
PR 37 13 2 1 1 54* Number of cells per high power field.† No expression noted.‡ Positive immunohistochemical reaction for respective receptor.
FIG. 1. Expression of estrogen receptor (ER) �. CREM, cremas-teric muscle. PROC�VAG, processus vaginalis. GUBERN, gubernac-ulum.
FIG. 2. Expression of progesterone receptor. ER, estrogen recep-tor. CREM, cremasteric muscle. PROC�VAG, processus vaginalis.GUBERN, gubernaculum.
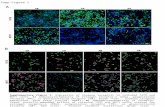
FIG. 3. Immunohistochemistry slides demonstrate different pat-terns of receptor expression. A, ER� expression in numerous cells ofcremaster muscle. H & E, reduced from �200. B, ER� expression insingle cells of processus vaginalis. H & E, reduced from �400. C, PRexpression in numerous cells of cremaster muscle. H & E, reducedfrom �400. D, PR expression in gubernaculum. H & E, reduced from�400.
EXPRESSION OF ESTROGEN AND PROGESTERONE RECEPTORS IN UNDESCENDED TESTIS1114
Estrogen receptors have been detected in various struc-tures, ie uterus, lungs, heart, seminal vesicles, bladder, pros-tate, suprarenal glands and testicles (Sertoli cells). However,their distribution and expression are tissue dependent andcan change with age.15 We have found no reports on estrogenand progesterone receptor expression in human paratesticu-lar structures. However, Barthold et al revealed the presenceof ER� in the gubernaculum of swine.16 Donaldson et alreported morphological changes in the gubernaculum of theER� knockout mouse and implicated a role of ER� in testic-ular descent.13
Our results demonstrate strong expression of estrogen re-ceptor in the gubernaculum, cremasteric muscle and proces-sus vaginalis obtained from boys with cryptorchidism. Inter-pretation of these results is difficult because of a lack ofsimilar studies in the literature. However, the number ofpatients in the control and cryptorchidism groups, andmarked and statistically significant differences in ER� andPR expression between groups, may be considered convincingevidence of the association between up-regulation of estrogenreceptor � and progesterone receptor and cryptorchidism.It is difficult to explain why ER� and progesterone receptor
would be over expressed in the paratesticular tissues of boyswith cryptorchidism without knowing serum estrogen levels.It has been shown that maternal or perinatal exposure toestrogen or xenoestrogen alters (imprints) expression of ER�in the prostate, gubernaculum and brain.16–18 One can as-sume that over expression of ER� and PR in paratesticularstructures may be secondary to local up-regulation of ER�and PR promoter during fetal development. Again, evidencefrom prostate revealed that the effect of fetal exposure toestrogens persists into adulthood.17 Barthold et al demon-strated an increased rate of cryptorchidism and an increasein ER� expression in the gubernaculum of swines exposed todioxin.16 This is an important study that supports a possibleassociation between ER� over expression and cryptorchid-ism. High density ER� and PR could modify transcription offactors important in testicular descent by changing relativeavailability of repressors and activators in the transcriptioncomplex. This result was shown by Zhang and Dufau, whofound orphan nuclear receptors binding to imperfect estrogenreceptor half site response element direct repeat locatedwithin the TATA-less promoter of the human LH receptorand affecting transcription of human LH receptor.11Down-regulation of insl-3 expression by estrogen may be
the most likely mechanism by which estrogens affect testic-
ular descent.6 This finding could suggest an important role ofestrogens in cryptorchidism.One should consider the harmful activity of environmental
progesterone and estrogen-like hormonal agents. The expres-sion of ER� and PR in investigated structures and unsatis-factory effects of androgen therapy in the treatment of cryp-torchidism can be results of increased exposure of humangonads to estrogens during pregnancy, although there arenot reliable experimental data on such interdependence.9The majority of our patients were born and have lived in anindustrial region of Lodz, Poland. Lodz is a major center ofthe textile and polymer industries, with known problems ofenvironmental pollution. We plan to extend our research andmeasure xenoestrogens together with endogenous estradiollevels in the blood of our patients in the future.It is possible that treatment with HCG in boys with cryp-
torchidism was responsible for increased expression of ER�.Experimental data show that HCG increases the number ofestrogen receptors but within 5 days of HCG/LH deprivationthe number of receptors decreases by half.19 Brinkmann et alfound that within 72 hours after discontinuation of HCG thenumber of estrogen receptors returns to normal.20 The factthat samples were obtained at least 6 months after treatmentwith HCGmay assure us that over expression of ER� and PRin boys with undescended testes is a primary defect and notcaused by treatment. We plan to investigate this area withfurther research, including boys without treatment withHCG.
CONCLUSIONS
Estrogen and progesterone receptors are expressed in tis-sues important for normal testicular descent. Our study re-vealed altered estrogen receptor � and progesterone receptorexpression in boys with undescended testes previouslytreated with HCG. The exact role that the estrogen andprogesterone receptors have in the descent of the testis is notclear. We hope that further research into the molecularmechanisms of cryptorchidism will enable us to provide bet-ter care and possibly prevention.
Drs. Eugene F. Fuchs, John M. Barry and Peter N. Schlegelassisted with preparation of the manuscript.
REFERENCES
1. Barthold, J. S. and Gonzalez, R.: The epidemiology of congenitalcryptorchidism, testicular ascent and orchiopexy. J Urol, 170:2396, 2003
2. Thonneau, P. F., Gandia, P. and Mieusset, R.: Cryptorchidism:incidence, risk factors, and potential role of environment; anupdate. J Androl, 24: 155, 2003
3. Carreau, S., Lambard, S., Delalande, C., Denis-Galeraud, I.,Bilinska, B. and Bourguiba, S.: Aromatase expression and roleof estrogens in male gonad: a review. Reprod Biol Endocrinol,1: 35, 2003
4. Emmen, J. M., McLuskey, A., Grootegoed, J. A. and Brinkmann,A. O.: Androgen action during male sex differentiation in-cludes suppression of cranial suspensory ligament develop-ment. Hum Reprod, 13: 1272, 1998
5. Husmann, D. A. and McPhaul, M. J.: Localization of the andro-gen receptor in the developing rat gubernaculum. Endocrinol-ogy, 128: 383, 1991
6. Zimmermann, S., Steding, G., Emmen, J. M., Brinkmann, A. O.,Nayernia, K., Holstein, A. F. et al: Targeted disruption of theInsl3 gene causes bilateral cryptorchidism. Mol Endocrinol,13: 681, 1999
7. Emmen, J. M., McLuskey, A., Adham, I. M., Engel, W.,Verhoef-Post, M., Themmen, A. P. et al: Involvement ofinsulin-like factor 3 (Insl3) in diethylstilbestrol-induced cryp-torchidism. Endocrinology, 141: 846, 2000
8. Tomboc, M., Lee, P. A., Mitwally, M. F., Schneck, F. X.,Bellinger, M. and Witchel, S. F.: Insulin-like 3/relaxin-likefactor gene mutations are associated with cryptorchidism.J Clin Endocrinol Metab, 85: 4013, 2000
FIG. 4. Estrogen receptor (ER) � is expressed in higher quantitiesin cryptorchidism (CR) and control (CON) groups compared to pro-gesterone receptor (PR). At same time PR is found in 16.7-fold morespecimens in cryptorchidism group than in controls. Numbers rep-resent average percentage of positive specimens in each group (gu-bernaculum, cremasteric muscle and processus vaginalis).
EXPRESSION OF ESTROGEN AND PROGESTERONE RECEPTORS IN UNDESCENDED TESTIS 1115
9. Norgil Damgaard, I., Main, K. M., Toppari, J. and Skakkebaek,N. E.: Impact of exposure to endocrine disrupters in utero andin childhood on adult reproduction. Best Pract Res Clin Endo-crinol Metab, 16: 289, 2002
10. Akazome, Y. and Mori, T.: Evidence of sex reversal in the gonadsof chicken embryos after oestrogen treatment as detected byexpression of lutropin receptor. J Reprod Fertil, 115: 9, 1999
11. Zhang, Y. and Dufau, M. L.: Nuclear orphan receptors regulatetranscription of the gene for the human luteinizing hormonereceptor. J Biol Chem, 275: 2763, 2000
12. Kuiper, G. G., Lemmen, J. G., Carlsson, B., Corton, J. C., Safe,S. H., van der Saag, P. T. et al: Interaction of estrogenicchemicals and phytoestrogens with estrogen receptor beta.Endocrinology, 139: 4252, 1998
13. Donaldson, K. M., Tong, S. Y., Washburn, T., Lubahn, D. B.,Eddy, E. M., Hutson, J. M. et al: Morphometric study of thegubernaculum in male estrogen receptor mutant mice. J An-drol, 17: 91, 1996
14. Damber, J. E., Bergh, A., Selstam, G. and Sodergard, R.: Estro-gen receptor and aromatase activity in the testes of the uni-lateral cryptorchid rat. Arch Androl, 11: 259, 1983
15. Greco, T. L., Furlow, J. D., Duello, T. M. and Gorski, J.: Immu-nodetection of estrogen receptors in fetal and neonatal malemouse reproductive tracts. Endocrinology, 130: 421, 1992
16. Barthold, J. S., Kryger, J. V., Derusha, A. M., Duel, B. P.,Jednak, R. and Skafar, D. F.: Effects of an environmentalendocrine disruptor on fetal development, estrogen receptoralpha and epidermal growth factor receptor expression in theporcine male genital tract. J Urol, 162: 864, 1999
17. Prins, G. S., Birch, L., Couse, J. F., Choi, I., Katzenellenbogen, B.and Korach, K. S.: Estrogen imprinting of the developing pros-tate gland is mediated through stromal estrogen receptor al-pha: studies with alphaERKO and betaERKO mice. CancerRes, 61: 6089, 2001
18. Tena-Sempere, M., Gonzalez, L. C., Pinilla, L., Huhtaniemi, I.and Aguilar, E.: Neonatal imprinting and regulation of estro-gen receptor alpha and beta mRNA expression by estrogen inthe pituitary and hypothalamus of the male rat. Neuroendo-crinology, 73: 12, 2001
19. de Boer, W., de Vries, J., Mulder, E. and van der Molen, H. J.:Comparative study of nuclear binding sites for oestradiol inrat testicular and uterine tissue. Determination of lowamounts of specific binding site by an [3H] oestradiol-exchange method. Biochem J, 162: 331, 1977
20. Brinkmann, A. O., Leemborg, F. G. and van der Molen, H. J.:hCG-induced inhibition of testicular steroidogenesis: anoestradiol-mediated process? Mol Cell Endocrinol, 24: 65, 1981
EXPRESSION OF ESTROGEN AND PROGESTERONE RECEPTORS IN UNDESCENDED TESTIS1116






















![RESEARCHARTICLE DefectiveResensitizationinHumanAirway … · 2016-11-11 · previously described [26].Although information concerningthe causeofdeath,gender, race andage ofthedonoris](https://static.fdocument.org/doc/165x107/5ea7317349d5e16b165d2f02/researcharticle-defectiveresensitizationinhumanairway-2016-11-11-previously-described.jpg)
