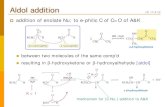
Sect. 1.5: Probability Distributions for Large N: (Continuous Distributions)
Evolution of Size Distributions with condensation of sulfuric acid on particles
description
Transcript of Evolution of Size Distributions with condensation of sulfuric acid on particles

1 Used Condensation Size Distribution Model from http://aerosols.ucsd.edu/aerosolsinmotion.html to determine the growth of the three distributions• Included the Condensation Rate Equation (Eq. 11.29
from Seinfeld and Pandis).2 Stevanovic et al., 2013 and Zhu et al., 2013 both had initial
distributions predominantly in Aitken and accumulation mode (appx. diameter 0.1 μm). The condensation of sulfuric acid did not affect the diameter size because the diameter was large relative to the amount of vapor condensed.
3 Su et al., 2013 measured particles in nucleation mode (diameter of 0.001-0.01 μm), thus the condensation of sulfuric acid had a greater affect on mean diameter size.
Evolution of Size Distributions with condensation of sulfuric acid on particles

Motivation: Coggon et al., 2012 measured marine aerosols and the affects of ship impacts during the E-PEACE campaign. Measurements of in cloud aerosols contained almost a 1:1 ratio of sulfate to the organic component (47% sulfate).
Approach: To simulate condensation of sulfuric acid onto particles, particles from initial size distributions were assumed to be composed of 100% organics and the Condensation Model was run.
Result: Particles emitted at <0.10 μm in diameter are composed of a larger fraction sulfuric acid than particles ≥0.10 μm in diameter.
Conclusions:
• The particles in the accumulation mode and larger half of Aitken mode are less affected by the condensation of sulfuric acid than particles in nucleation mode.
• Su et al., 2013 contained particles in nucleation and Aitken mode, thus it took approximately 8 days to grow particles to a 1:1 ratio of sulfuric acid to organics.
• Zhu et al., 2011 and Stevanovic et al., 2013 contained particles in the upper bound of Aitken mode and accumulation mode, however, even after condensation of sulfuric acid persisted for 24 days, the ratio of sulfuric acid to organics did not reach 1:1, but rather 0.65:1.
Evolution of Size Distributions with condensation of sulfuric acid on particles
Reference Paper Time to condense sulfuric acid to 50% of initial particle massStevanovic et al., 2013 >576 hours (24 days)
Zhu et al., 2011 >576 hours (24 days)Su et al., 2013 194-195 hours (~8days)