Evidence for Enzymatic Catalysis of the Diels-Alder Reaction in Nature
Transcript of Evidence for Enzymatic Catalysis of the Diels-Alder Reaction in Nature

Evidence for Enzymatic Catalysisof the Diels-Alder Reaction in Nature
Carmen DrahlSorensen Group
Organic Supergroup Literature PresentationJuly 2005

Laschat, S. Angew Chem. Int. Ed. 1996, 35, 289.Nicolaou, K. C.; Sorensen, E. J. in Classics in Total Synthesis, 1996, 265-267.
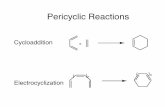
Does Nature Know the Diels-Alder Reaction?
CO2R
Ph
H H
H
H
H
Ph
HCO2H
representative member of enediandric acids
CO2
OHCO2
O2C
OH
O
CO2[3, 3] sigmatropic rearrangement
chorismate mutase
i. 8π conrot.ii. 6π disrot.iii. π4s + π2s

Nelson, D. L.; Cox, M. M. in Lehninger Principles of Biochemistry, 3rd ed. 2000, 246-250.
What is an Enzyme? Thermodynamic Review
S
P
Transition State ‡
∆G‡S->P
∆G‡P->S
∆G'°
Reaction Coordinate
Free
Ene
rgy,
G
S
P
Transition State ‡
∆G‡uncat
Reaction Coordinate
Free
Ene
rgy,
G
ES EP
‡
∆G‡cat
∆G'° = −RT ln K'eq
Catalysts do NOT affect reaction equilibria.Catalysts enhance reaction rates by lowering activation energies.
k = Ae -∆G‡/RT

O
O OCH3
O
H
H
solanapyrone A
Solanapyrone A: decalin polyketide phytotoxin produced by the pathogenic fungus Alternaria solani, causal organism of potato early blight disease
Solanapyrone A inhibits DNA polymerase β and γ.
The first synthesis of (±)-solanapyrone A was achieved through an intramolecular Diels-Alder reaction, which fueled speculation about its assembly in Nature.
Ichihara, A.; Tazaki, H.; Sakamura, S. Tet. Lett. 1983, 24, 5373Ichihara, A.; Miki, M.; Tazaki, H.; Sakamura, S. Tet. Lett. 1987, 28, 1175.Mizushina, Y., et al. J. Biol. Chem. 2002, 277, 630-638.
Solanapyrone A
O
O OCH3
SS
180 °C, sealed tube
toluene, 1 h, 71%O
O OCH3
SS
O
O OCH3
SS
+
H
HH
H2 endo : 1 exo

Oikawa, H.; Yokota, T.; Ichihara, A.; Sakamura, S. J. Chem. Soc. Chem. Comm. 1989, 1284.Oikawa, H.; Suzuki, Y.; Naya, A.; Katayama, K.; Ichihara, A. J. Am. Chem. Soc. 1994, 116, 3605.
Solanapyrone D and a Proposed Biosynthesis
OO
OCH3O
exo endo
OO
OCH3O
O
O OCH3
O
H
H
solanapyrone A (MAJOR)
O
O OCH3
O
H
H
solanapyrone D
The proposed triene precursor contains no chiral centers.Solanapyrones A and D are found in nature in 100% ee.

Oikawa, H.; Yokota, T.; Abe, T.; Ichihara, A.; Sakamura, S.; Yoshizawa, Y.; Vederas, J. C. J. Chem. Soc. Chem. Comm.1989, 1282.
Solanapyrones: Feeding Experiment Summary
O
O OCH3
O
H
H
solanapyrone A
O
OCHO
OCH3
D
D
O
OD
DD
D
D D
2 [CH3-13C]met
D
DD
R
O
R
O
R
O
OO
R
R =O
O OCH3
O solanapyrone A
X
Michael Aldol
Diels Alder
8
retention of D at C5!

Oikawa, H.; Suzuki, Y.; Naya, A.; Katayama, K.; Ichihara, A. J. Am. Chem. Soc. 1994, 116, 3605.
* Feeding experiments with III inconclusive. III underwent spontaneous endo cyclization in aqueous conditions.
Biosynthesis: Summary of Feeding Experiments
8 acetate+
2 C1-Met
O
O OCH3
O
O OCH3
O
O OCH3
OH O
prosolanapyrone II
prosolanapyrone IIII
prosolanapyrone III *III
O
O OCH3
O
H
H
solanapyrone A
O
O OCH3
HO
H
H
solanapyrone B
O
O OCH3
O
H
H
solanapyrone D
O
O OCH3
HO
H
H
solanapyrone E
Intramolecular Diels-Alder
[O][O]
++2H +2H

Oikawa, H.; Kobayashi, T.; Katayama, K.; Suzuki, Y.; Ichihara, A. J. Org. Chem. 1998, 63, 8748.
Solution Reactivity of Prosolanapyrones
O
OR
OCH3
R = CH3 (I)
R = CH2OH (II)
R = CHO (III)
Endo-selectivity increases with increasing solvent polarity.
Rate depends on the oxidation level of the pyrone substituent.
substrate solvent temperature (°C) time (h) yield (%) SM recovery (%) endo /exoI PhCH3 180 48 12 11 1.9I H2O 30 168 7 93 > 10II PhCH3 110 48 55 2 2.2II CHCl3 110 2 7 91 3.6II CH3CN 110 24 71 18 5.6II H2O 30 48 19 81 20III PhCH3 110 1 68 27 2.7III CHCl3 110 1 64 28 3.4III CH3CN 110 1 82 10 4.4III H2O 30 3 62 28 23

Oikawa, H.; Katayama, K.; Suzuki, Y.; Ichihara, A. J. Chem. Soc. Chem. Comm. 1995, 1321.Katayama, K.; Kobayashi, T.; Oikawa, H.; Honma, M.; Ichihara, A. Biochim. et Biophys. Acta, 1998, 387.An enantioselective synthesis of solanapyrone A utilized this crude enzyme for the IMDA:Oikawa, H.; Kobayashi, T.; Katayama, K.; Suzuki, Y.; Ichihara, A. J. Org. Chem. 1998, 63, 8748.
Solanapyrone Synthase: Improved Exo Selectivity
O
OR
OCH3 R = CH3 (I)
R = CH2OH (II)
R = CHO (III)
O
O OCH3
O
H
H
O
O OCH3
O
H
H
solanapyrone A (exo)solanapyrone D (endo)
+
Crude enzyme preparation oxidized II to III. No reaction of II in absence of O2(g).Background uncatalyzed cyclization of III competes with enzymatic reaction, hencelower ee when III is used as starting material.Enzyme not yet isolated; further purification of the enzyme(s) responsible is in progress.
substrate conditions yield (%) SM recovery (%) endo :exo ee (%)II control 0 100 n/aII + extract 19 75 + 6 % of III 0.176 99III control 15 85 32.3III + extract 25 75 0.887 92III + denatured extract 10 90 32.3

Lovastatin (mevinolin) is produced by fermentation of the fungal strain Aspergillus terreus, and has also been isolated from Monascus ruber.
The lactone opened form is a potent inhibitor of the liver enzyme HMG-CoA reductase, which reduces HMG-CoA to mevalonate, the rate limiting step in cholesterol biosynthesis.
Prescribed as Mevacor (Merck) to lower cholesterol and fats in blood.
No bicyclic precursor less oxidized than dihydromonacolin L has been reported.
Alberts, A. W., et al. Proc. Natl. Acad. Sci. U.S.A. 1980, 77, 3957.Endo, A. J. Antibiot. 1979, 32, 852.Endo, A. Trends Biochem. Sci. 1981, 6, 10.Kennedy, J.; Auclair, K.; Kendrew, S. G.; Park, C.; Vederas, J. C.; Hutchison, C. R. Science 1999, 284, 1368.
Lovastatin
O
O
O
OHO
OHCOOH
O
O
HO
lovastatin (closed form) lovastatin (open form)
O
OHO
dihydromonacolin L

Chan, J. K.; Moore, R. N.; Nakashima, T. T.; Vederas, J. C. J. Am. Chem. Soc. 1983, 105, 3334.Moore, R. N.; Bigam, G.; Chan, J. K.; Hogg, A. M.; Nakashima, T. T.; Vederas, J. C. J. Am. Chem. Soc.1985, 107, 3694.Yoshizawa, Y.; Witter, D. J.; Liu, Y. Q.; Vederas, J. C. J. Am. Chem. Soc. 1994, 116, 2693.
Lovastatin: Feeding Experiment Summary
O
O
O
OHO
O
OO
H3C
O
OD
DD
9 + 2 2 [CH3-13C]metD
DD
O*
O
D
D
DH3CD D D
DD
D
Diels-Alder proposed as key biogenesis step:
O SR O SR
H
lovastatin

Witter, D .J.; Vederas, J. C. J. Org. Chem. 1996, 61, 2613.
Lovastatin: Attempted Laboratory Cyclizations
O X
a, X = S(CH2)2NHAcb, X = OEtc, X = OH
X
O
O
X
endo exo
XO O
X
endo exo
X
O X
H
H
O X
H
H
O X
H
H
O X
H
H
lovastatin
Thermal: 1 : 1 a,b,c(EtAlCl2): 9 : 1 b 19 : 1 a
Not observed.Is an enzyme needed tostabilize pseudoaxial Me?

Witter, D .J.; Vederas, J. C. J. Org. Chem. 1996, 61, 2613.
Solution Reactivity of Lovastatin Precursor
O X
a, X = S(CH2)2NHAcb, X = OEtc, X = OH
O X
H
H
O X
H
H
endo exo
(Transition states for pseudoequatorial methyl)+
substrate conditions temperature (°C) time (h) yield (%) SM recovery (%) endo /exoa PhCH3 160 96 81 n/a 1b PhCH3 160 96 72 6 1c PhCH3 160 96 83 n/a 1a EtAlCl2 + PhCH3 23 3 80 n/a 9b EtAlCl2 + PhCH3 23 3 58 n/a 19b CHCl3 22 240 50 n/a n/ab H2O:CH3CN:MeOH (5:5:1) 28 48 50 n/a n/a

Witter, D .J.; Vederas, J. C. J. Org. Chem. 1996, 61, 2613.
Lovastatin: Attempted Feeding Experiment
O SNHAc
O
O
O
OHO
lovastatin (closed form)
X
Feeding this 13C labeled substrate to Aspergillus terreus resulted in no formation of carbon-carbon coupled lovastatin or precursors.Presumably, the substrate is catabolized before it can undergo cycloaddition.

Auclair, K.; Sutherland, A.; Kennedy, J.; Witter, D. J.; Van den Heever, J. P.; Hutchinson, C. R.; Vederas, J. C.J. Am. Chem. Soc. 2000, 122, 11519.
LNKS Enzyme Affords Correct Stereochemistry
O X
a, X = S(CH2)2NHAcb, X = OEt
O X
H
H
O X
H
H
+
O X
H
H
1 2 3natural product's stereochemistry
Stereochemistry found in the natural product (3) is only obtainable in the presence ofpurified LNKS enzyme. The enzyme may stabilize the transition state through van der Waals or other contacts.kcat = 0.073 ± 0.001 min-1. Nonenzymatic cyclization competes with catalysis.
+

Sakurai, I.; Suzuki, H.; Miyaijima, K.; Akiyama, S.; Simizu, S.; Yamamoto, Y. Chem. Pharm. Bull. 1985,33, 5141.Oikawa, H.; Yagi, K.; Watanabe, K.; Honma, M.; Ichihara, A.; Chem. Commun. 1997, 97.
Macrophomate: Benzoate from a Pyrone
O
O
OCH3
O
HO2C OCH3
O
- 2CO2; - H2O
OO
O
OO macrophomic acid
Isolated from Macrophoma commelinae fungus, which causes spots on the leaves of the Asiatic dayflower.
The benzoate macrophomate is made by an unusual multistep transformationfrom a 2-pyrone. This type of aromatic compound is typically biosynthesizedvia the shikimate or polyketide pathway.
http://www.sycamoreisland.org

R2
Oikawa, H.; Yagi, K.; Watanabe, K.; Honma, M.; Ichihara, A. Chem. Commun. 1997, 97.
Macrophomate: Summary of Biosynthetic Studies
OCO
OCH3
OOCH3
Omacrophomic acid
HO O
R1
OH
R2OH
OH
HOCO2H
H2N H
CO2HMe
H2N H
*
^
glycerolR1, R2 = H or D
[1-13C]-L-serine
[1-13C]-L-alanine
OO
O
O O
oxaloacetate
*
+
O
O
OPO32-
O
O
Opyruvate
phosphoenolpyruvate
Oxaloacetate was determined to be the most likely three-carbon donor by isotopeincorporation.
5-acetyl-4-methoxy-6-methyl-2-pyrone
^
- CO2, -H2O

Watanabe, K.; Mie, T.; Ichihara, A.; Oikawa, H.; Honma, M. J. Biol. Chem. 2000, 275, 38393.
Michael/Aldol or Diels-Alder?
O
O
H3CO
O
OO
O
OO
O
O
H3CO
O HCO2
HOH
O O
O
OO
O OO
OCH3 O
OO
OCH3O
OO
O- CO2
- CO2
OCH3
O
HO
O- CO2, H2O

Oikawa, H.; Yagi, K.; Watanabe, K.; Honma, M.; Ichihara, A. Chem. Commun. 1997, 97.
A Bicyclic Inhibitor of Macrophomate Synthesis
O
O
OCH3
O
OCH3
O
HO
O
OO
O
O O
+ O
O
MeO
O HCO2
HOH
proposed transition state forDiels-Alder reaction
i. oxaloacetate decarboxylationii. Diels-Alder reaction
cell-free extract M. commelinae
O
O
MeO
O

Sato, H.; Konoma, K.; Sakamura, S. Agric. Biol. Chem. 1979, 43, 2409.Sato, H.; Konoma, K.; Sakamura, S. Agric. Biol. Chem. 1981, 45, 1675.Ichihara, A.; Murakami, K.; Sakamura, S. Tetrahedron 1987, 43, 5245.
Biomimetic Synthesis of Pyrenochaetic Acid A
O
O OCH3
O
HO2C OCH3
O
Pyrenochaeta terrestrisor
Macrophoma commelinae
O
O OCH3
O
O
OEtO
O
OMe
O
X
Y
+180-200 °C, sealed tube
neat, 17 h, 84%
pyrenocine A pyrenochaetic acid A
HO2C OCH3
O
macrophomic acid
-CO2
X OCH3
OY
Product 1 closely resemblesmacrophomic acid. product 1:
X = CO2Et; Y = Hproduct 2:X = H; Y = CO2Et1:2 ratio = 2:3

Ose, T.; Watanabe, K.; Mie, T.; Honma, M.; Watanabe, H.; Yao, M.; Oikawa, H.; Tanaka, I. Nature 2003, 422, 185.Berman, H. M. et al. The Protein Data Bank. Nucl. Acids Res. 2000, 28, 235.Image rendered with Deep View / Swiss PDB Viewer. http://www.expasy.org/spdbv/
Crystal Structure of Macrophomate Synthase
Structure 1.7 Å in complex with pyruvate and Mg2+.
MW = 36 kDa.Hexameric functional unit associated by hydrophobic interactions.
Each protomer is an 8-stranded β-barrel containing an octahedrally coordinatedMg2+ ion. Magnesium was known at the time to be necessary for oxaloacetatedecarboxylation.

Ose, T., et al. Nature 2003, 422, 185.Watanabe, K.; Oikawa, H.; Yagi, K.; Ohashi, S.; Mie, T.; Ichihara, A.; Honma, M. J. Biochem. 2000, 127, 467.Berman, H. M. et al. The Protein Data Bank. Nucl. Acids Res. 2000, 28, 235.Image rendered with Deep View / Swiss PDB Viewer. http://www.expasy.org/spdbv/
Macrophomate Synthase Active Site
The Mg2+ ion stabilizes the pyruvate enolate.Enzyme kcat= 0.60±0.02 s-1
Arg101 and Tyr169 are thought to bind pyrone. Mutants lose MPS activity while retainingdecarboxylase activity.Steric congestion of peptide backbone allows access to one face of enolate.Product inhibition is avoided bysecond decarboxylation anddehydration.

Guimarães, C. R. W.; Udier-Blagovic, M.; Jorgensen, W. L. J. Am. Chem. Soc. 2005, 127, 3577.Wilson, E. Chem. Eng. News. 2005, 83(18), 38.
"A Bucket of Cold Water"
O
O
H3CO
O
O O
O
OO
O OO
OCH3 O
OO
OCH3O
OO
O
17.7 kcal/mol 12.1 kcal/mol
Crystal structure provided the basisfor a QM/MM/ Monte Carlocalculations to investigate the energetics of both possible pathways.The Diels-Alder transition state is significantly less stable than bothMichael and Aldol transition states.

Pohnert, G. ChemBioChem 2003, 4, 713.Stocking, E. M.; Williams, R. M. Angew. Chem. Int. Ed. 2003, 42, 3078.Pitt, J. N.; Ferré-D'Amaré, A. R. Nat. Struct. Mol. Biol. 2005, 12, 206.
Diels-Alderases: Summary
— Each of the three putative Diels-Alderases catalyzesone or several reactions prior to the cyclization step.solanapyrone synthase: oxidationlovastatin nonaketide synthase: polyketide chain formationmacrophomate synthase: decarboxylation
—General strategy appears to be entropy trapping of the substratein the correct conformation to facilitate a [4+2] cycloaddition.
—"Proof that the proteins are accelerating the rates of the pericyclicDiels-Alder reaction remains to be rigorously established".(RNA Diels-Alderases have shown up to 20,000 fold rate enhancement).
—"Calculations as well as work with mutants and inhibitors will haveto clarify to what extent the Diels-Alder reaction in the enzymeactive site of macrophomate synthase does indeed follow aconcerted... pathway."

Carey, F. A.; Sundberg, R. J. in Advanced Organic Chemistry, 4th ed. 2000, 222-225.
Kinetic Isotope Effect and Concerted Mechanisms
Kinetic Isotope Effect: difference in reaction rate when an atom is replacedby its isotope
R-HR-D
En = hν (n + ½)
ν = 1/2π sqrt(k/µ)

Belasco, J. G.; Albery, W. J.; Knowles, J. R. J. Am. Chem. Soc. 1983, 105, 2475.
Kinetic Isotope Effect and Stepwise Mechanisms
Isotopic fractionation at the bond-making site measured as a function of the isotope at the bond-breaking site can be used to test for concertedness of enzymatic mechanism. Example: the enzyme proline racemase.
2-1H - D-proline L-proline
H"BB-H' B-H"B-H' B-H"B H'
2-2H - D-proline L-proline
lose H" lose D"gain D'
gain H'
gain D'
gain H'

Belasco, J. G.; Albery, W. J.; Knowles, J. R. J. Am. Chem. Soc. 1983, 105, 2475.
Kinetic Isotope Effect and Concerted Mechanisms
Isotopic fractionation at the bond-making site measured as a function of the isotope at the bond-breaking site can be used to test for concertedness of enzymatic mechanism.
H"BB-H' B-H"B H'
lose D", gain D'lose D", gain H'lose H", gain D'lose H", gain H'
D-proline L-proline

Key Papers: Diels-Alder Reactions in Biology
Biosynthetic Diels-Alder Reactions (established and speculated)— Stocking, E. M.; Williams, R. M. Angew. Chem. Int. Ed. 2003, 42, 3078.— Oikawa, H.; Tokiwano, T. Nat. Prod. Rep. 2004, 21, 321.— Oikawa, H. Bull. Chem. Soc. Jpn. 2005, 78, 537.
Antibody Diels-Alder Catalysis— Hilvert, D.; Hill, K. W.; Nared, K. D.; Auditor, M. M. J. Am. Chem. Soc. 1989, 111, 9261.— Romesberg, F. E.; Spiller, B.; Schultz, P. G.; Stevens, R. C. Science 1998,279, 1929.— Heine, A., et al. Science 1998, 279, 1934.
RNA Diels-Alder Catalysis— Tarasow, T. M.; Tarasow, S. L.; Eaton, B. E. Nature 1997, 389, 54.— Seelig, B.; Jäschke, A. Chem. Biol. 1999, 6, 167.

Meinwald, J.; Hwang, H. C.; Christman, D.; Wolf, A. P. J. Am. Chem. Soc. 1960, 82, 483.
Problem: Mechanism?
Propose a mechanism for the acid catalyzed rearrangement of cinenic acid.
OH3CH3C CH3
conc. H2SO4
OH3CCO2H
CH3
CH3
OOH

Meinwald, J.; Hwang, H. C.; Christman, D.; Wolf, A. P. J. Am. Chem. Soc. 1960, 82, 483.
Problem: Mechanism?
Propose a mechanism for the acid catalyzed rearrangement of cinenic acid.
OH3CH3C CH3
conc. H2SO4
OH3CCO2H
CH3
CH3
OOH
OH3CH3C CH3
OOH2
OH3CH3C CH3
O
OH3CH3C CH3
OH3CH3C CH3
OH3CH3C
CH3
OH3CH3C
CH3
O
H+
- H2O
- CO
+ CO
+ H2O
By decarboxylation, cinenic acid can rid itself of a 1,3diaxial interaction (either between 2 methyls or a methyl
and a carboxyl).





![Index [application.wiley-vch.de] · Index a Abbasov/Romo’s Diels–Alder lactonization 628 ab initio – calculations 1159 – molecular orbital calculations 349 – wavefunction](https://static.fdocument.org/doc/165x107/5b8ea6bc09d3f2a0138dd0b3/index-index-a-abbasovromos-dielsalder-lactonization-628-ab-initio.jpg)