Enteric nerve fibers of holothurians are recognized by an antibody to acetylated α-tubulin
Transcript of Enteric nerve fibers of holothurians are recognized by an antibody to acetylated α-tubulin
Neuroscience Letters, 157 (1993) 153-156 153 © 1993 Elsevier Scientific Publishers Ireland Ltd. All rights reserved 0304-3940/93/$ 06.00
NSL 09648
Enteric nerve fibers of holothurians are recognized by an antibody to acetylated -tubulin
Jos6 E. Garcia-Arrarfis and Eduardo Viruet
Department of Biology, University of Puerto Rico, Rio Piedras Campus, Rio Piedras, PR 00931 (USA)
(Received 29 January 1993; Revised version received 6 April 1993; Accepted 12 April 1993)
Key words: Echinoderm; Enteric nervous system; Invertebrate neurobiology; Microtubule; Nerve fiber
The distribution of immunoreactivity to 6-11B-l, a monoclonal antibody that labels acetylated ~-tubulin, was studied in the radial nerve and intestinal system of holothurians. As shown previously for other species, this antibody recognizes cilia and nerve fibers in Holothuria glaberrima and Holothuria mexicana. Thus, anti-acetylated a-tubulin can be used as a marker for nerve fibers in the enteric nervous system.
The regeneration of the nervous system in echino- derms has been the focus of experiments in our labora- tory. As a model, we are using holothurians, or sea cu- cumbers, in which regeneration of the digestive system occurs following the evisceration process [10]. To study neuronal regeneration and, in our case, regeneration of the enteric nervous system, it is necessary to have mark- ers of nervous tissue. The ideal markers should recog- nize, with high specificity, molecules associated with nerve fibers and/or neuronal cell bodies. For example, they might recognize the presence of neuroactive sub- stances, neuronal membrane antigens, or a subset of cy- toskeletal filaments present in neurons, among others. A putative marker of neuronal fibers is the presence of the acetylated posttranslational modification of ct-tubulin. This modified form of tubulin is an important compo- nent of the microtubule-organizing center of flagella and cilia, and immunoreactivity to this molecule has been found to be present in different cellular structures, in- cluding axonemes, basal bodies and centrioles [11,13,14]. However, expression of acetylated ct-tubulin is not con- fined to these structures but has also been found to be preferentially expressed in nerve fibers, both in vivo and in vitro [14,12]. Moreover, experiments have shown that acetylated ct-tubulin is an important component of neurons undergoing growth-related processes [2,6].
Correspondence: J.E. Garcla-Arrar~is, Department of Biology, Univer- sity of Puerto Rico, Rio Piedras, PR 00931, USA. Fax: (1) (809) 765- 9695.
We have used a monoclonal antibody that recognizes unequivocally the acetylated ct-tubulin molecule to deter- mine if neuronal fibers in the holothurians Holothuria glaberrima and H. mexicana can be labelled by im- munocytochemistry. Organisms were collected from the north coast of Puerto Rico and were mantained at the facilities of the Institute of Neurobiology of the Univer- sity of Puerto Rico. After dissection of organisms, per- formed as described previously [7,9], pieces of large and small intestine and radial nerve were fixed in picric acid- formaldehyde (Zamboni) fixative, dehydrated by an al- cohol series and sectioned in a cryostat. 10-tim sections were used for indirect immunocytochemistry using con- ditioned culture media of the 6-11B-I hybridoma that recognizes the acetylated ~-tubulin molecule [13]. Sec- tions were incubated with a dilution of 1/2-1/10 of the cell-culture supernatant. The following day, sections were rinsed with phosphate-buffered saline (PBS), incu- bated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin (Tago, Burlingame, CA) di- luted 1/50 for 1 h, rinsed again and mounted in buffered glycerol (pH = 8.6).
In addition, double-labelling experiments were done to determine if the immunoreactivity to acetylated ct-tu- bulin coexisted with previously described fibers express- ing neuropeptides. Procedures for double-labelling were similar to those published previously [7], except that the primary antibody mixture consisted of the rabbit anti- neuropeptide [see 9] and the culture supernatant of the anti-acetylated ~-tubulin at final dilutions of 1/1000 and 1/10, respectively. The secondary antibody mixture was
154
rhodamine-conjugated goat anti-rabbit (Tago) and FITC-conjuga ted goat anti-mouse (Tago). Appropia te
controls were performed [see 8] and no cross-reactivity was observed among the sera. Sections were observed with a Leitz Laborlux fluorescence microscope.
The holothur ian nervous system consists o f five radial
nerves that join together in the anterior part o f the ani- mal to form a central nerve ring. Each nerve is divided into an ectoneural component , with both sensory and
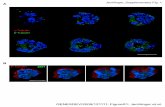
moto r functions, and a hyponeural component , with solely mo to r functions [10]. Radial nerve sections f rom H. glaberrima showed strong fluorescence in the ectoneu- ral and hyponeural components (Fig. 1). The im-
munofluorescence was found th roughout the radial nerve, particularly in the neuropile, and was observed
mainly as fluorescent dots or specks probably originat- ing f rom fibers that had been sectioned transversaly. However, some longitudinaly sectioned fibers were clearly visible. Cell bodies on the other hand were not
Fig. 2. lmmunohistochemistry of acetylated ct-tubulin in a transverse section of the large intestine of H. ,~laberrima. The labelled fibers (ar- rows) are mainly found surrounding the longitudinal muscle (LM), no tiber is observed within the circular muscle (CM). Immunoreactive cilia
(arrowheads) are observed bordering the lumen (L/. Bar, 5 #m.
Fig. 1. lmmunohistochemistry to acetylated ct-tubulin in a transverse section of the radial nerve of H. glaberrima. Immunoreactivity is found in the fibers that form the neuropile of the ectoneural (El and hyponeu- ral (H) components but not in the basal lamina (BL) that separates them. In addition, the cilia of the epithelial cells that border the coelo-
mic cavity are strongly labelled by the antibody. Bar, 40/am.
immunoreact ive and, in many instances, could be recog- nized as dark areas outlined by the surrounding fluores-
cent fibers. No immunoreact ivi ty was present in the
basal lamina separating the two nervous system compo- nents (Fig. 1). Lateral nerves extending from the radial
nerve were also immunofluorescent in a manner similar to that described for the radial nerve. Excluding the nerv- ous component , strong immunoreact ivi ty was present only in filiform structures surrounding the coelomic cav- ity, probably originating in the ciliated epithelium.
The small and large intestines of H. glaberrima and H.
mexicana were tested for immunoreact ivi ty to the acetyl- ated ~-tubulin antibody. The results obtained from large
or small intestine o f both holothur ian species are essen- tially identical and will be presented without distinction. The holothurian intestinal system, as described by H y m a n [10], can be divided into five layers. The outer
two, collectively named the serosa, consist of a single-cell layer that lines the celomic border and the outer connec- tive tissue layer. Moving toward the lumen, one encoun- ters the muscular layer, the submucosa or internal con- nective tissue layer and the mucosal epithelium layer which faces the lumen.
All tissue layers except for the mucosa, contain struc- tures that express immunoreact ivi ty to acetylated ~-tu- bulin (Fig. 2), These structures are all of a filiform mor- phology and nowhere were cell bodies or any type o f cellular morpho logy recognized by the antisera. The im- mune reaction is strongest in the coelomic epithelial lin-
155
ing where individual filamentous structures of close to 20 tim in length can be observed all along the periphery of the intestine (Fig. 2). These immunoreactive structures, similar to those observed in the radial nerve sections, are probably the cilia known to exist in the epithelial cells that line the coelomic cavity [10]. A fiber plexus, contain- ing material immunoreactive to the monoclonai anti- body, can be found within the muscular layer (Fig. 2). This plexus is made of fibers that are thinner in width and weaker in their immune reaction than the coelomic cilia; however, they are extremely numerous and were observed to surround almost completely the longitudinal muscle fibers. Few, if any, fibers were observed within the circular muscle layer, although occasionally, fibers cross the circular muscle layer and penetrate the submu- cosal space. In the submucosa, long immunoreactive fi- bers were seen. Most of these fibers run perpendicular to the muscle layer and apparently traverse the submucosal layer from the muscle to the mucosal layer. The fibers rarely branch within the submucosal space and are only infrequently observed to run longitudinal to the muscu- lar layer.
The distribution of ~x-tubulin-like immunoreactivity closely parallels that of the neuropeptides cholecystoki- nin and FMRFamide [7~9]. To verify that the acetylated ~-tubulin is really labelling enteric nervous tissue, we performed double-labelling experiments. These experi- ments showed that fibers expressing immunoreactivity to GFSamide (the neuropeptide responsible for F M R F a immunoreactivity in sea cucumbers [see 5]) and to CCK also express immunoreactivity to acetylated ~x-tubulin (Fig. 3). This coexpression was observed in the fiber plexus within the serosal-longitudinal muscle area and was clearly visible in the fibers within the submucosal layer. However, not all structures expressing the acetyl- ated ~x-tubulin coexpressed the peptide, several fibers within the serosal, muscular and submucosal layer only expressed the acetylated ~-tubulin immunoreactivity. Of particular importance is the fact that the strongly fluo- rescent cilia of the coelomic epithelium did not express immunoreactivity to the peptides (Fig. 3A,D, arrow- heads).
Microtubules are an important element in the func- tional cytoskeleton of neurons and play a prominent role in the maintenance and growth of nerve fibers [2,6,11]. They are assembled from c~ and fl tubulin subunits. The acetylated form of ~-tubulin, a posttranslational modifi- cation of the e-amino group of lysines, plays a key role in their stabilization [3,13,14]. 16-11 B-l, a monoclonal an- tibody raised against tubulin from the axonemes of sea urchin sperm flagella, is known to recognize the acetyl- ated form of ~-tubulin of a variety of organisms [13~14]. Using this antibody, the molecule has been found to be
preferentially expressed in cilia, flagella and nerve fibers [1-4,12-14]. Its specific localization within the nervous system has been a controversial subject since limited lo- calization to axons has been reported by one group [4] while other authors have localized the molecule to both axons and dendrites [1,12].
The pattern of the immunereaction in the holothurian tissues correlates well with the previously described anti- body specificity [13]. First, the antibody is known to stain flagella and cilia and in our hands it labelled very strongly the cilia of the coelomic epithelium. This la- belling also serves as an internal control for the double- labelling experiments since it clearly shows that not all fibrillar structures labelled by the monoclonal antibody are labelled by the polyclonal anti-CCK sera. Secondly, the antibody is known to label nerve fibers [4,12]. In this respect, the pattern and distribution of fibers labelled with the anti-acetylated ~ tubulin in the serosa, muscle
Fig. 3. Double-labelling of fibers in the large intestine of H. glaberrima with (A,C) acetylated cc-tubulin, (B) GFS. and (D) CCK antibodies. Most but not all the fibers expressing acetylated ~-tubulin also express neuropeptide immunoreactivity (arrows). The fibers are shown in both the longitudinal muscle (A,B) and in the submucosa (C,D). Notice that the cilia (arrowhead) are not labelled by the peptide antisera. *Cell in submucosa expressing endogenous fluorescence. Bar. 15 (A,B) and 5
pm (C.D)
156
and submucosa, is identical with the pattern obtained with two antibodies that label neuropeptides [7,9] and, in addition to the pattern observed at the electron micro- scope level, for vesicle-containing fibers (K. Rivera, un- publ. data). The similarity of the labelling patterns is confirmed by the double-labelling experiments which show that neuropeptide-containing fibers are also recog- nized by the anti-acetylated ~-tubulin antibody. Cell bodies are not labelled, resembling results obtained in the mammalian cerebellum where only axons are labelled [4]. Whether in the holothurians acetylated c~-tubulin is limited to axons or is found in both axons and dendrites is difficult to determine, particulary in view that very little is known about the neuronal circuitry of the enteric system of these organisms. However, it is reasonable to propose that at least axons are being labelled, particu- larly those surrounding the longitudinal muscle. These should correspond to motor axons controlling intestinal contraction. In addition, we have previously postulated that the long fibers that traverse the submucosal space are axons from the neurons found in the serosa.
Our results show that the 16-11B-I monoclonal anti- body labels, at least partially, the nervous system of holothurians and its staining pattern corresponds to the neuronal network previously described by our group [9]. Thus, it can serve as a marker in our study of nervous system regeneration.
We thank G. Piperno, Rockefeller University, New York, for his generous gift of the 6--11B-I antibody. We also acknowledge the excellent technical support of I. Torres-Avill/m and L.B. Jimrnez. This work was sup- ported by grants from the Whitehall Foundation and the University of Puerto Rico FIPI Program. In addition, we acknowledge the support of NIH-MBRS (RR-8102-18), NSF (BNS-8801538) and the Department of Biology, University of Puerto Rico.
l Baas, P.W. and Black, M.M., Individual microtubules in the axon consist of domains that differ in both composition and stability, J. Cell Biol., 111 (1990) 495-509.
2 Baas, P.W. and Heidemann, S.R., Microtubule reassembly from nucleating fragments during the regrowth of amputated neurites, J. Cell Biol., 103 (1986) 917-927.
3 Black, M.M., Cochran, J.M. and Kurdyla, J.T., Solubility proper- ties of neuronal tubulin: evidence for labile and stable microtubules, Brain Res., 295 (1984) 255-263.
4 Cambray-Deakin, M.A. and Burgoyne, R.D, Postranslational modifications of tubulins: acetylated and detyrosinated forms in axons of rat cerebellum, J. Cell Biol., 104 (1987) 1569-1574.
5 Diaz-Miranda, L., Price, D.A., Greenberg, M.J., Lee, T.D., Doble, K.E. and Garcia-Arrar~s, J.E., Characterization of two novel neu- ropeptides from the sea cucumber Holothuria glaberrima, Biol. Bull., 182 (1992) 241-247.
6 Ferreira, A. and C~ceres, A., The expression of acetylated microtu- bules during axonal and dendritic growth in cerebellar macroneu- rons which develop in vitro, Dev. Brain Res., 49 (1989) 205-213.
7 Garcia-Arrar~s, J.E., Enamorado-Ayala, I., Torres-Avill~n, I. and Rivera, V., FMRFamide-like immunoreactivity in cells and fibers of the holothurian nervous system, Neurosci. Lett., 132 ( 1991) 199- 202.
8 Garcia-Arrar/ts, J.E. and Martlnez, R., Developmental expression of serotonin-like immunoreactivity in the sympathoadrenal system of the chicken, Cell Tiss. Res., 262 (1990) 363 372.
9 Garcla-Arrar/ts, J.E., Torres-Avill~in, I. and Ortiz-Miranda, S., Cells in the intestinal system of holothurians (Echinodermata) ex- press cholecystokinin-like immunoreactivity, Gen. Comp. Endocri- nol., 83 (1991) 233-242.
10 Hyman, L.H., The Invertebrates: Echinodermata, Vol. 4, McGraw- Hill, New York, 1967.
11 Mohri, H. and Hosoya, N., Two decades since the naming of tu- bulin; the multi-facets of tubulin, Zool. Sci., 5 (1988) 1165-1185.
12 Morales, M. and Fifkov/t, E., Distribution of acetylated tubulin in brain, in situ localization and biochemical characterization, Cell Tiss. Res., 265 (1991)415423.
13 Piperno, G. and Fuller, M.T., Monoclonal antibodies specific for an acetylated form of tubulin recognize the antigen in cilia and flagella from a variety of organisms, J. Cell Biol., 101 (1985) 2085- 2094.
14 Piperno, G., LeDizet, M. and Chang, X., Microtubules containing acetylated tubulin in mammalian cells in culture, J. Cell Biol., 104 (1987) 289-302.