Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor...
Transcript of Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor...

Biochimica et Biophysica Acta 1826 (2012) 370–384
Contents lists available at SciVerse ScienceDirect
Biochimica et Biophysica Acta
j ourna l homepage: www.e lsev ie r .com/ locate /bbacan
Review
Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis byoncogenes and tumor suppressors in cancer cells
Jin-Qiang Chen a,b,⁎, Jose Russo a
a Breast Cancer Research Laboratory, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USAb Gold-Belt Falcon LLC, 860 Greebrier Circle, Suite 410, Chesapeake, VA 23360, USA
⁎ Corresponding author at: Breast Cancer Research LaE-mail address: [email protected] (J.-Q. Chen).
0304-419X/$ – see front matter © 2012 Elsevier B.V. Aldoi:10.1016/j.bbcan.2012.06.004
a b s t r a c t
a r t i c l e i n f oArticle history:Received 27 April 2012Received in revised form 16 June 2012Accepted 18 June 2012Available online 27 June 2012
Keywords:p53c-MycGlucose transporterGlycolysisTCA cycleGlutaminolysis
A common set of functional characteristics of cancer cells is that cancer cells consume a large amount of glu-cose, maintain high rate of glycolysis and convert a majority of glucose into lactic acid even in the presence ofoxygen compared to that of normal cells (Warburg's Effects). In addition, cancer cells exhibit substantial al-terations in several energy metabolism pathways including glucose transport, tricarboxylic acid (TCA) cycle,glutaminolysis, mitochondrial respiratory chain oxidative phosphorylation and pentose phosphate pathway(PPP). In the present work, we focused on reviewing the current knowledge about the dysregulation of theproteins/enzymes involved in the key regulatory steps of glucose transport, glycolysis, TCA cycle andglutaminolysis by several oncogenes including c-Myc and hypoxia inducible factor-1 (HIF-1) and tumor sup-pressor, p53, in cancer cells. The dysregulation of glucose transport and energy metabolism pathways by on-cogenes and lost functions of the tumor suppressors have been implicated as important biomarkers forcancer detection and as valuable targets for the development of new anticancer therapies.
© 2012 Elsevier B.V. All rights reserved.
Contents
1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3712. p53, C-Myc and HIF-1 involved in the regulation of glucose transport, glycolysis, TCA cycle, and glutaminolysis . . . . . . . . . . . . . . . 371
2.1. p53 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3712.2. c-Myc . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3712.3. HIF-1 and its regulation by SIRT3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 372
3. Regulation of glucose transporters in cancer cells by p53 and HIF-1α . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3723.1. Glucose transporter systems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3723.2. Regulation of glucose uptake and GLUT-1 expression by GSK-3/TSC2/mTOR/AKT . . . . . . . . . . . . . . . . . . . . . . . . . . 3733.3. Negative regulation of GLUT-1, GLUT-3 and GLUT-4 by p53 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3733.4. Regulation of glucose transport by HIF-1α . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3733.5. GLUT-1 as a potentially important anti-cancer target . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 374
4. Dysregulation of enzymes/protein factors involved in glycolysis in cancer cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3744.1. Regulation of hexose kinases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3744.2. Regulation of phosphofructokinase by TIGAR, HIF-1 and posttranslational modifications . . . . . . . . . . . . . . . . . . . . . . . 375
4.2.1. Regulation of PFK-1 by TP53-induced glycolytic and apoptotic regulator (TIGAR) . . . . . . . . . . . . . . . . . . . . . . 3754.2.2. Regulation of PFK-1 by HIF-1α . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3754.2.3. Posttranslational modifications of PFK1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 376
4.3. Dysregulation of pyruvate kinase and pyruvate dehydrogenase in cancer cells . . . . . . . . . . . . . . . . . . . . . . . . . . . 3764.3.1. Dysregulation of pyruvate kinase . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3764.3.2. Regulation of pyruvate dehydrogenase . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 376
5. Altered expression/functions of enzymes/tumor suppressors involved TCA cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3775.1. TCA cycle in cancer cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3775.2. Mutations of genes that encode enzymes in TCA cycle in cancer cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 377
5.2.1. Mitochondrial aconitase in cancer cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 377
boratory, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA. Tel.: +1 443 824 3532.
l rights reserved.

371J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
5.2.2. Mutations of IDH1 in cancer cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3775.2.3. Mutations of SDHD in cancer cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3785.2.4. Mutations of FU in cancer cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 378
6. Regulation of glutaminolysis by C-Myc and p53 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3796.1. Glutamine and glutaminolysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 379
6.1.1. Regulation of glutamine transport and glutaminase-1 by c-Myc . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3796.2. Regulation of GLS2 by p53 and GLS2 as an potential target for cancer therapy . . . . . . . . . . . . . . . . . . . . . . . . . . . 379
7. Conclusion remarks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 380Acknowledgements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 380References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 380
1. Introduction
Cancer cells exhibit a common set of functional characteristics,i.e. they consume a larger amount of glucose, maintain amuch higherrate of glycolysis and convert a majority of glucose into lactic acideven in the presence of oxygen compared to that of normal cells.This phenomenon was first described over 70years ago [1,2] andhas been known as the Warburg's Effect (aerobic glycolysis) [3].The tumor cells preferentially use glycolysis over mitochondrial oxi-dative phosphorylation for glucose-dependent ATP production. Thisdeviant energetic metabolism is a potential hallmark of cancer cellsand has been thought to be the root of tumor formation and growth[4]. While the mechanisms underlying the Warburg's Effects havenot been completely understood, a number of oncogenes includingC-Myc and hypoxia inducible factors-1 (HIF-1) and tumor suppres-sors such as p53 have been known to be involved in the regulationof energy metabolism (for review see [5,6]). The notable factors cru-cial for cancer metabolic phenotype are oncogenic mutations thatalter growth factor signaling through the Phosphoinositide 3-kinase(PI3K)/Akt (Protein Kinase B, PKB)/themammalian target of rapamycin(mTOR) pathway [7]. Activation of this pathway enhancesmetabolic ac-tivities of glycolysis by two major events. First, the synthesis of thesugar transporter GLUT-1 is induced to facilitate glucose uptake by thecells [6,8,9]. Second, the activity of transcription complex HIF-1α is in-creased, which in cooperationwith transcription factor c-Myc enhancesthe synthesis of the majority of glycolytic enzymes [10]. The distinctphenotype of high glucose uptake in cancer cells has important clin-ical implications in that it can be documented by positron emissiontomography (PET) scanning of human cancers with radiolabeled2-deoxyglucose and 18F-fluorodeoxy-glucose [11,12].
Based on Warburg's hypothesis, glycolysis is predominately usedin cancer cells because of a dysregulation of mitochondrial oxidativephosphorylation. However, not all cancers are PET-positive, and notall models of neoplastic transformation are associated with in-creased aerobic glycolysis. While hypoxic cells exhibit a shift towardglycolytic metabolism [13], a functional mitochondrial respiratorychain and a glutamine-derived carbon are required for proliferationof most transformed cells [14]. It has been known that most cancercells do not have defects in mitochondrial metabolism [2], exceptfor rare mutations in succinate dehydrogenase (SDH) or fumaratehydratase (FH), both are enzymes of the tricarboxylic acid (TCA)cycle [6,15,16]. The oncogene c-Myc is known to be involved notonly in regulation of glycolysis but also stimulates mitochondrialbiogenesis [17] and glutamine catabolism [6,18,19]. Furthermore, aproteomic study of breast cancer brain metastases detected in-creases in expression of enzymes/proteins involved in glycolysis,TCA cycle, oxidative phosphorylation and pentose phosphate path-ways (PPP) [20]. Consistent with this, it has been observed that met-abolic changes accompanying transformation and acquisition ofmetastatic potential in a syngeneic mouse mammary tumor model[21] and in human triple negative breast cancer cell lines MCF-10Fseries [22] included changes not only in the key metabolites inglycolysis but also in TCA cycle, PPP, and fatty acid/nucleotide bio-synthesis. These observations indicate the need of rethinking the
prevailing models of cancer metabolism, particularly if these alter-ations are exploited for therapeutic purposes [6,11].
Oncogenes and tumor suppressors have been linked to the regu-lation of glucose and energy metabolism, thereby connecting geneticalterations in cancers to their glucose metabolic phenotype [23,24].A number of the metabolic changes can be attributed to the activa-tion and/or malfunction of oncogenes, and/or loss of tumor suppres-sors. In this work, we focused on reviewing the current knowledgeabout the dysregulation of glucose transport, glycolysis, TCA cycleand glutaminolysis by tumor suppressor p53 and oncogenes, c-Mycand HIF-1α, in cancer cells, and then pointed out the potential impli-cations of these biomarkers/targets for cancer detection and for thedevelopment of new anticancer therapies.
2. p53, C-Myc and HIF-1 involved in the regulation of glucosetransport, glycolysis, TCA cycle, and glutaminolysis
2.1. p53
p53, a well studied tumor suppressor, plays critical roles in the con-trol of a number of cellular processes including apoptosis, cell cycle ar-rest, genomic stability, and angiogenesis. In its anti-cancer role, p53works through several mechanisms. It can initiate apoptosis, if DNAdamage proves to be irreparable and it can induce growth arrest byholding the cell cycle at the G1/S regulation point on DNA damage rec-ognition [25,26]. p53 mainly exerts its tumor suppression functionthrough the transcriptional regulation of its target genes. Upon its acti-vation induced by oxidative stress, p53 binds to DNA and induces theexpression of several different sets of genes including those involvedin cell cycle, apoptosis, DNA repair and oxidative stress response [25].Notably, activation of the expression of the cyclin-dependent kinase in-hibitor p21WAF1/CIP1 by p53 plays an important role in induction of G1cell cycle arrest [27,28]. Another important function of p53 is its in-volvement in the regulation of intracellular reactive oxygen species(ROS) levels, playing an important role in determining the death or sur-vival of cells [29]. p53 can activate numerous genes that results in in-creased generation of ROS, which contribute to apoptosis [27]. It alsofunctions in a feedback loop in which ROS can signal to further activa-tion of p53 [30]. On the other hand, p53 can induce the expression ofproteins that function to lower ROS levels and this antioxidant functionof p53 is important in preventing DNA damage and tumor developmentunder low stress conditions [31]. Recent studies have revealed a num-ber of new functions of p53 in the regulation of glucose metabolismand energymetabolism pathways including glucose transport [32], gly-colysis [33], TCA cycle [34], glutaminolysis [35,36], mitochondrial respi-ratory chain/oxidative phosphorylation [37] and PPP [38,39], whichwillbe described below.
2.2. c-Myc
The proto-oncogene c-Myc encodes a transcription factor involvedin many cellular processes, including proliferation, cell cycle progres-sion, cell growth, metabolism, angiogenesis, differentiation, cell adhe-sion, and mobility primarily through transcriptional regulation of

372 J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
large gene networks that ultimately results in activation or repressionof target genes. Because of its broad regulatory scope, the expressionof the c-Myc gene itself needs to be tightly controlled. Dysregulated ex-pression and/or malfunction of c-Myc are one of the most common ab-normalities in human malignancy. The c-Myc is involved in regulationof a large number of genes involved in the biogenesis of ribosomes andmitochondria, and regulation of glucose and glutamine metabolism aswell as microRNAs (miRNA). With E2F1, c-Myc induces genes involvedin nucleotide metabolism and DNA replication, and microRNAs thathomeostatically attenuate E2F1 expression. With the HIF-1, ectopicc-Myc cooperatively induces a transcriptional program for hypoxic ad-aptation. The c-Myc regulates gene expression either directly, such asglycolytic genes including lactate dehydrogenase A (LDHA) [40]. Thec-Myc gene is frequently activated in many different human cancers,which are highly dependent on sustained c-Myc expression whereasc-Myc inactivation results in desirable anticancer effects, such as celldeath, differentiation, and/or senescence. Thus, c-Myc has emerged asan attractive target for cancer therapy [41,42].
In addition to its known functions mentioned above, recent stud-ies have documented a new role for c-Myc in stimulating glutaminecatabolism, in part through activation of proteins necessary for cellsto engage in glutamine catabolism [11,40,43] (see Section 6.1.1).
2.3. HIF-1 and its regulation by SIRT3
Hypoxia is the result of an imbalance between oxygen deliveryand oxygen consumption, causing a reduction of oxygen tensionbelow the normal level for a specific tissue. Hypoxia is a common fea-ture of solid tumors and is associated with their malignant phenotype[44]. HIF-1 is a basic–helix–loop–helix transcription factor that playsessential roles in mammalian development and physiology. HIF-1 isa heterodimer composed of HIF-1α and HIF-1β subunits. The expres-sion and activity of the HIF-1α subunit are tightly regulated by cellu-lar O2 concentration. The levels of α subunit increase during hypoxiawhereas the β subunit is constitutively expressed. The HIF-1α subunithas been used as a marker for hypoxia. HIF-1 is essential for embryonicvascularization/survival, neovascularization in ischemic myocardium,hypoxia-induced pulmonary vascular remodeling, and tumor vascular-ization. HIF-1α is over-expressed in the majority of common humancancers and their metastases, due to the presence of intratumoral hyp-oxia and as a result of mutations in genes encoding oncoproteins andtumor suppressors [45]. It has been known that HIF-1α is involved inthe control of the expression of genes involved in glucose transport[46], glycolysis [45,47], TCA cycle [48,49] and glutaminolysis [11].
The sirtuin gene (SIRT) family is the mitochondrial NAD-dependentdeacetylase that mediates metabolic reprogramming by destabilizingHIF-1α [50,51]. It has been shown [51,52] that SIRT3 loss increasesROS production, leading to HIF-1α stabilization. SIRT3 expression is re-duced in human breast cancers and several other malignancies and itsloss correlates with the up-regulation of HIF-1α target genes. Loss ofSIRT3 results in aberrantmitochondrial metabolism and genomic insta-bility, while its over-expression represses glycolysis and proliferation inbreast cancer cells, providing a metabolic mechanism for tumor sup-pression [50]. Primary mouse embryo fibroblasts or tumor cell lines ex-pressing SIRT3 short-hairpin RNA exhibited a greater potential toproliferate, and augmented HIF-1α protein stabilization and transcrip-tional activity in hypoxic conditions. Knocking down SIRT3 increases tu-morigenesis in mouse xenograft models, and this is abolished by theanti-oxidant, N-acetyl cysteine. Moreover, over-expression of SIRT3 in-hibits stabilization of HIF-1α protein in hypoxia and attenuates in-creases in HIF-1α transcriptional activity. Critically, over-expression ofSIRT3 decreased tumorigenesis in xenografts, even when induction ofthe sirtuin occurred after tumor initiation. These data suggest thatSIRT3 acts to suppress the growth of tumors, in part, through its abilityto suppress ROS and HIF-1α [51].
3. Regulation of glucose transporters in cancer cells by p53 andHIF-1α
3.1. Glucose transporter systems
Glucose enters cells via a family of twelve functional glucose trans-porters (GLUTs), designated GLUT-1 to GLUT-12. The majority ofwhich are tissue-specific, for example, GLTU-1 (all tissues but abun-dance in brain and erythrocyte), GLUT-2 (liver), GLUT-3 (brain),GLUT-4 (muscle/fat), and GLUT-5 (small intestine) [53]. GLUTs are inte-gral membrane proteins that contain 12 membrane-spanning heliceswith both the amino and carboxyl termini exposed on the cytoplasmicside of the plasma membrane. GLUT proteins transport glucose and re-lated hexoses according to a model of alternate conformation, whichpredicts that the transporter exposes a single substrate binding site to-ward either the outside or the inside of the cell [54–56]. Binding of glu-cose to one site provokes a conformational change associated withtransport, and releases glucose to the other side of the membrane. Theinner and outer glucose-binding sites are located in transmembranesegments 9, 10, 11 [57] and the glutamine–leucine–serinemotif locatedin the seventh transmembrane segment could be involved in the selec-tion and affinity of transported substrate [58,59].
Among the GLUTs, GLUT-1 is a rate-limiting transporter for glu-cose uptake, and its expression correlates with anaerobic glycolysis.GLUT-1 has an influence not only on glucose uptake/utilization butalso on tumorigenic features, including metastasis, chemoresistanceand escape from immune surveillance. Increased expression ofGLUT-1 has been observed in breast, lung, colorectal cancers and pri-mary hepatocellular carcinoma (HCC) [53,60–63].
Higher GLUT-1 expression by breast cancer cells compared withthe healthy breast tissue is common [60,62]. Brown et al. [60] exam-ined the expression of GLUT-1, GLUT-2, GLUT-3, GLUT-4 and GLUT-5in paraffin sections from 12 primary human breast cancers and8 lymph node metastases from 2 patients. They detected GLUT-1 inall the primary breast cancers and the lymph node metastaseswhere GLUT-1 was expressed on the cell membrane and in the cyto-plasm of the tumor cells, but exhibited marked intratumoral andintertumoral variability in the proportions of positive cells and the in-tensity of staining. Its expression was much lower in the normalmammary epithelium than in tumor cells from the same patient.While GLUT-2 was expressed in all of the tumors, its staining intensi-ty was not consistently stronger than that seen in healthy breast.Clusters of GLUT-4‐positive granule were observed in cells in six ofthe tumors but none of the tumors or the healthy breast in thetissues tested expressed GLUT-3 or GLUT-5.
Grover-McKay et al. [61] observed that cell surface GLUT-1 expres-sion was associated with the increasing invasive ability of the humanbreast cancer lines MCF-7, MDA-MB-435 and MDA-MB-231. However,GLUT-2 and GLUT-5 were inversely associated with invasiveness;GLUT-3 expression was variable; and GLUT-4 was undetected. In apoorly differentiated human ductal breast cancer, in situ GLUT-1staining was intense. Higher GLUT-1 expression was also observed ina large fraction of colorectal carcinomas from the patients. The patientswith higher GLUT-1 staining were 2.3 times that in the group with lowGLUT-1 staining GLUT-1. The expression was significantly correlatedwith female gender, non-mucinous tumor type, poorer differentiation,and lymph nodemetastasis [62,63]. HCC is one of themost fatal cancersin humans with rising incidence in many regions around the world. Ithas been shown [64,65] that the GLUT-1 expression is increased in asubset of HCC patients and functionally affects tumorigenicity. RNAinterference-mediated inhibition of GLUT-1 expression in HCC cellsresulted in reduced tumorigenicity whereas over-expression ofGLUT-1 in bladder, breast, cervical, colorectal, gastric, lung and thy-roid tumors is associated with metastasis and/or poor prognosis ofthese tumors (for review see [53]). Furthermore, increased expres-sion of GLUT-1 protein is correlated with the increased uptake of

373J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
2-[18F]-fluoro-2-deoxy-D-glucose (FDG) by several types of tumorsobserved by PET imaging [66–68]. These findings suggest thatGLUT-1 could be a therapeutic target for these cancers.
3.2. Regulation of glucose uptake and GLUT-1 expression byGSK-3/TSC2/mTOR/AKT
The expression and localization of glucose transporters are highlyregulated in several ways dependent on the cell type and the stimuliinvolved. Several studies [69–71] have shown that glycogensynthase kinase-3 (GSK-3)/tuberous sclerosis complex subunit 2(TSC2)/the mammalian target of rapamycin (mTOR) pathway isone of the important pathways involved in the regulation of glucosetransport.
GSK-3 (GSK-a/3β), an important signaling molecule that is ubiqui-tously expressed, has been implicated in the regulation of glucosetransport and metabolism. GSK-3 is known to function downstreamof phosphatidylinositol-kinase (P13K) and AKT [72]. Akt/PKA (Pro-tein Kinase B) is a serine/threonine-specific protein kinase thatplays a key role in multiple cellular processes such as glucose metab-olism, apoptosis, cell proliferation, transcription and cell migration.The tuberous sclerosis complex subunit 2 (TSC2) is a putative tumorsuppressor, which has been shown to interact with GSK-3 [73].mTOR is a serine/threonine protein kinase that regulates cell growth,cell proliferation, cell motility, cell survival, protein synthesis, andtranscription. mTOR belongs to PI3K related kinase protein family[74,75]. mTOR integrates the input from upstream pathways, includ-ing insulin, growth factors (such as IGF-1 and IGF-2), and amino acids[74]. mTOR also senses cellular nutrient and energy levels and redoxstatus [76].
Buller et al. [71] have provided several lines of evidence indicatingthat GSK-3/TSC2/mTOR pathway is involved in regulation of GLUT-1in several cell types. First, chronic inhibition of basal GSK-3 activityin several cell types, including vascular smooth muscle cells, resultedin increase in glucose uptake due to a similar increase in protein ex-pression of GLUT1while expression of a constitutively active form ofGSK-3β resulted in decrease in GLUT1 expression and glucose uptake;Second, GSK-3 inhibits mTOR signaling via phosphorylation of TSC2and the absence of functional TSC2 resulted in increase in glucose up-take and GLUT1 expression in multiple cell types, which wereprevented by inhibition of mTOR with rapamycin. The effect ofGSK-3 inhibition on GLUT1 expression and glucose uptake was re-stored in TSC2mutant cells by transfection of a wild-type TSC2 vector,but not by a TSC2 construct with mutated GSK-3 phosphorylationsites. Third, GSK-3 inhibition had no effect on glucose uptake orGLUT-1 expression in TSC2 mutant cells. These results indicate thateffects of GSK-3 on GLUT-1 and glucose uptake are mediated by aTSC2/mTOR-dependent pathway. GSK-3 suppresses glucose uptakevia TSC2 and mTOR and may serve to match energy substrate utiliza-tion to cellular growth. It has also reported that HIF-1α levels wereenhanced through an mTOR-dependent mechanism in cells derivedfrom TSC2 negative mouse embryo fibroblast [77]. These studies sug-gest that TSC2 is an important negative regulator of GLUT-1 expres-sion and glucose uptake. Inactivating mutations in TSC2 likely leadto enhanced GLUT-1 expression and basal glucose uptake in anon-insulin-sensitive manner [71]. On the other hand, GLUT-1 wasfound to enhance mTOR activity independently of TSC2 and AMPK[78].
It has been known that Akt/PKB is required for insulin-stimulatedglucose transport in skeletal muscle and adipose cells. Zhou et al. [70]have revealed a link between Akt activation and glucose transport reg-ulation. Using proteomic and bioinformatic approaches, they identifiedthe Rab GAP (GTPase-activating protein)-domain containing proteinTBC1D1 [TBC (Tre-2/Bub2/Cdc16) domain family, member 1], which isclosely related to TBC1D4 [TBC domain family, member 4], as an Aktsubstrate. RNA interference (siRNA)-mediated silencing of TBC1D1
elevated basal deoxyglucose uptake by approx. 61% in 3T3-L1 mouseembryo adipocytes, while the suppression of TBC1D4 under thesame conditions had little effect on basal and insulin-stimulateddeoxyglucose uptake. Silencing of TBC1D1 strongly increased ex-pression of GLUT1 glucose transporter but not GLUT4 in cultured ad-ipocytes, whereas the decrease in TBC1D4 had no effect. Loss ofTBC1D1 in 3T3-L1 adipocytes activated the mTOR–p70 S6 protein ki-nase pathway, and the increase inGLUT-1 expression in the cells treatedwith TBC1D1 siRNAwas blocked by themTOR inhibitor rapamycin. Fur-thermore, over-expression of the mutant TBC1D1-T590A, lacking theputative Akt/PKB phosphorylation site, inhibited insulin stimulation ofp70 S6 kinase phosphorylation, which is induced by mTOR. These datasuggest that TBC1D1may be involved in controlling GLUT-1 expressionthrough themTOR–p70 S6 kinase pathway. Akt regulates the transcrip-tion [79] and translation (through mTOR) and eukaryotic translationinitiation factor 4E binding protein 1 (4E-BP1) [80] of GLUT-1. BecausethemTORpathway is dysregulated in certain cancers [75], its regulatoryrole on GLUT-1 might be lost.
3.3. Negative regulation of GLUT-1, GLUT-3 and GLUT-4 by p53
It has been shown that p53 is involved in down-regulation ofglucose transport genes [32,81]. Schwartzenberg-Bar-Yoseph et al.[32] transiently co-transfected osteosarcoma-derived SaOS-2 cells,rhabdomyosarcoma-derived RD cells, and C2C12 myotubes withGLUT1-P-Luc or GLUT4-P-Luc promoter-reporter constructs andwild-type p53 expression vectors. They observed that p53 expres-sion dose dependently decreased both GLUT-1 and GLUT-4 promot-er activity to approximately 50% of their basal levels. The inhibitoryeffect of wild-type p53 was greatly reduced or abolished when cellswere transfected with p53 with mutations in amino acids 143, 248,or 273. A region spanning −66/+163bp of the GLUT-4 promoterwas both necessary and sufficient to mediate the inhibitory effectsof p53. Furthermore, in vitro translated p53 protein was found tobind directly to two sequences in that region and p53–DNA bindingwas completely abolished by excess unlabeled probe but not bynonspecific DNA, and was super-shifted by the addition of ananti-p53 antibody. These data suggest that wild-type p53 repressesGLUT-1 and GLUT-4 gene transcription in a tissue-specific manner.Mutations within the DNA-binding domain of p53, which are usual-ly associated with malignancy, were found to impair the repressiveeffect of p53 on transcriptional activity of the GLUT-1 and GLUT-4gene promoters, thereby resulting in increased glucose metabolismand cell energy supply.
Kawauchi et al. [81] have found a link between p53, the transcrip-tion factor NF-κB and glycolysis. In p53-deficient primary culturedcells, kinase activities of IKKα and IKKβ and subsequent NF-κB activ-ity were enhanced. Activation of NF-κB by loss of p53 caused an in-creased rate of aerobic glycolysis and up-regulation of GLUT3.Oncogenic Ras-induced cell transformation and acceleration of aero-bic glycolysis in p53-deficient cells were suppressed in the absenceof p65/NF-κB expression, and were restored by GLUT3 expression. Itwas also shown that a glycolytic inhibitor diminished the enhancedIKK activity in p53-deficient cells. Moreover, in Ras-expressingp53-deficient cells, IKK activity was suppressed by p65 deficiency andrestored by GLUT3 expression. These data indicate that p53 restricts ac-tivation of the IKK–NF-κB pathway through suppression of glycolysis,suggesting the presence of a positive-feedback loop exists, whereby gly-colysis drives IKK–NF-κB activation, and that hyperactivation of this loopby loss of p53 is important in oncogene-induced cell transformation.
3.4. Regulation of glucose transport by HIF-1α
Several studies [46,82–84] have shown that hypoxia induced anelevated expression level of GLUT-1 via HIF-1α. Chondrocytes residein a hypoxic environment and utilize glucose as the main energy

374 J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
source. Ren et al. [82] have demonstrated that hypoxia enhanced ex-pression levels of GLUT-1 and GLUT-3 via HIF-1α in chondrocytes. Incontrast, deletion of HIF-1α led to complete loss of thehypoxia-induced expression of GLUT-1 and GLUT-3. Similarly, in-duction of GLUT-1 expression in BeWo choriocarcinoma cells [84]and in trophoblast-derived cells [46] was found to be mediated byHIF-1α. HIF-1 has been known to play a key role in the repro-gramming of cancer metabolism by activating transcription of anumber of genes encoding glucose transporters as well as glycolyticenzymes (for review see [13]).
3.5. GLUT-1 as a potentially important anti-cancer target
Since GLUT-1 is over-expressed and confers poor prognosis in awide range of solid tumors, GLUT-1 has been proposed as a potential-ly important target for development of anti-cancer drugs. [64,85,86].More recently, Liu et al. [87] identified a small molecule inhibitor ofGLUT-1 namedWZB117 that was shown to be effective not only in in-hibition of cell growth in cancer cell lines but also in inhibition of can-cer growth in a nude mouse model. Daily intraperitoneal injection ofWZB117 at 10mg/kg resulted in an over 70% reduction in the size ofhuman lung cancer of A549 cell origin through decreasing the levelsof GLUT-1 protein, intracellular ATP, and glycolytic enzymes. Thesechanges were followed by increase in ATP-sensing enzyme AMPKand declines in cyclin E2 as well as phosphorylated retinoblastomaprotein, resulting in cell cycle arrest, senescence and necrosis. Thisstudy points to the possibility of using WZB117 as a prototype for de-velopment of anticancer therapeutics targeting GLUT-1-mediatedglucose transport and glucose metabolism.
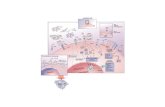
Fig. 1. Regulation of glucose transporters, glycolysis by GSK-3/(TSC2)/(mTOR)/AKT, C-Myc, HGLUT1–4: glucose transporters 1–4; mTOR: the mammalian target of rapamycin; PFK1: phoberous sclerosis complex subunit 2.
4. Dysregulation of enzymes/protein factors involved in glycolysisin cancer cells
Glycolysis is the integrate center of primary metabolism pathways.The rate of glucose utilization via the glycolytic pathway is highly regu-lated and depends upon the energetic andmetabolic needs of the cell. Itis coordinated with other pathways of energy generation and utiliza-tion, including gluconeogenesis, PPP, and the TCA cycle [88].
Glycolytic flux in eukaryotic organisms is tightly controlled by allo-steric enzymes that retain their regulation by feedback inhibition inspite of the elevated activities of intermediary enzymes. This statementis supported by the experiments in Escherichia coli [89] and Saccharomy-ces cerevisiae [90], where over-expression of all glycolytic enzymes hadno significant effect on the rate of glucose consumption. Therefore, it islikely that important modifications of the kinetics of regulatory en-zymes are also involved in metabolic changes that occur during thetransformation of normal mammalian cells into cancer cells [91]. Gly-colysis is tightly regulated by three key allosteric enzymes: hexokinase(HK), 6-phosphofructo-1-kinase (PFK1) and pyruvate kinase (PK). Eachcatalyzes individual irreversible step (Fig. 1). These enzymes, in turn,are tightly regulated by a number of oncogenes and tumor suppressorsand the normal control of these enzymes appears to be impaired intumor cells.
4.1. Regulation of hexose kinases
When it enters the cells via glucose transporters, hexose (glucoseor fructose) is phosphorylated by hexose kinases (HK) (glucose ki-nase or fructose kinase) to a hexose phosphate (glucose-phosphate
IF-1 and p53 HIF-1α: hypoxia inducible factor 1α; GSK-3: glycogen synthase kinase-3;sphofructokinase-1; TGAR: TP53-induced glycolytic and apoptotic regulator; TSC2: tu-

375J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
or fructose phosphate). HK is the first regulatory step of glycolysisand is feedback inhibited by its product, glucose-6-phosphate (G6P).
There are four important mammalian HK isozymes designatedHKI, HKII, HKIII and HKIV. They vary in subcellular locations and ki-netics with respect to different substrates and conditions, and physi-ological functions. HKI is found in all mammalian tissues and isconsidered a “housekeeping enzyme,” unaffected by most physiolog-ical, hormonal, and metabolic changes. HKII is the principal isoformregulated in many cell types. HKIII is substrate-inhibited by glucoseat physiologic concentrations. Mammalian HKIV (also known as glu-cokinase) differs from other HKs in kinetics and functions. The loca-tion of the phosphorylation on a subcellular level occurs whenglucokinase translocates between the cytoplasm and nucleus of livercells. HKIV can only phosphorylate glucose if the concentration ofthis substrate is high enough because its Km for glucose is 100times higher than that of other three HKs. It is monomeric, about50kDa, displays positive cooperativity with glucose, and is not allo-sterically inhibited by G6P. It is present in the liver, pancreas, hypo-thalamus, small intestine, and perhaps certain other neuroendocrinecells, and plays an important regulatory role in glucose metabolism.
Among the HKs, HKII is the predominant isoform in tumor cellswhere it is bound to the mitochondrial outer membrane facing thecytosol [92]. Microlocation of this enzyme enables its preferential ac-cess to newly synthesized ATP for phosphorylating glucose, and it isresistant to product inhibition [92]. The association of HKII with mito-chondria is activated by Akt, and the mitochondria-associated HKII isinvolved in inhibition of apoptosis [93,94] through its binding to thevoltage-dependent anion channel, negatively modulating truncatedBH3-interacting domain death agonist and perhaps to BCL1 antago-nist of cell death (BAD) [95,96]. HKII promoter contains functionallyactive response elements for p53 and is up-regulated by p53 [97].Mitochondrially-bound HKII is thought to be a key player in thegrowth and survival of many cancers and an ideal prospect for thera-peutic intervention because drugs that disassociate HKII from mito-chondrial membrane caused apoptosis and interfered with growthpathways [98].
4.2. Regulation of phosphofructokinase by TIGAR, HIF-1 andposttranslational modifications
6-Phosphofructo-1-kinase (PFK1) catalyzes the phosphoryla-tion of fructose-6-phosphate (F6P) to fructose-1, 6-bisphosphate(Fru-1,6-P2) using MgATP as a phosphoryl donor [99] (Fig. 1). PFKcatalyzes the first irreversible, rate-limiting step and the most im-portant control point unique to the glycolytic pathway, which sur-mounts the regulatory roles of the other two allosteric enzymes[100]. PFK1 is stimulated by ADP/AMP whereas citrate and ATPact as strong inhibitors [99,101]. Therefore, glycolysis is slowedwhen cellular ATP concentrations are high. ATP binds to a site onPFK distinct from the active site, causing a conformational changedue to rotation of the positions of Arg162 and Glu161. In thehigh-affinity state, the positive charge on Arg162 stabilizes the neg-ative charge on the phosphate of F6P, and Km is low. In thelow-affinity state, the negative charge on Glu161 repels F6P.
Fructose-2,6-bisphosphate (F-2,6-BP) is a powerful allostericregulator for controlling carbon flux through glycolysis by stimu-lating PFK to convert F6P to F1,6-P2. The synthesis and degradationof F-2,6-P2 depend upon 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFK-2/F-2,6-BPase), which has both kinase andphosphatase activities [102–104]. This bifunctional enzyme is reg-ulated by phosphorylation and dephosphorylation that are depen-dent upon intracellular cAMP levels [105]. Furthermore, PFK-2/F-2,6-BPase synthesis can be induced by mitogens, growth factors,and inflammatory cytokines, implicating its role in setting the glyco-lytic rate under multiple physiologic and pathologic conditions [106].This inducible gene for PFK-2 is expressed constitutively in several
human cancer cell lines and is found to be required for tumor cellgrowth. Inhibition of inducible PFK-2 protein expression decreases theintracellular level of 5-phosphoribosyl-1-pyrophosphate, a product ofthe PPP and an important precursor of purines and pyrimidines fornucleic acid biosynthesis [106].
Four different genes coding different isozymes (PFKFB1–4) havebeen identified to date [107–110]. PFKFBs 1–4 differ not only in theirtissue distribution but also in their kinetic and regulatory properties.The PFKFB3 isozyme has the highest kinase/phosphatase activity ratioand thus maintains elevated F-2,6-P2 levels, which in turn sustainshigh glycolytic rates [106,111]. Significantly, this isoform is constitu-tively expressed in several human cancer cell lines having high prolifer-ative rates that require the elevated activity of the enzyme for thesynthesis of 5-phosphoribosyl-1-pyrophosphate [106,112,113]. The ex-pression of PFKFB is highly elevated inmultiple aggressive primary neo-plasms, including colon, breast, ovarian, and thyroid carcinomas and isinduced by hypoxia in cultured human colon adenocarcinoma cells.Furthermore, PFKFB3 and Fru-2,6-P2 levels increase specifically duringS phase of the cell cycle in normal lung fibroblasts [114]. Thus, thiscould explain the high glycolytic rates present in transformed cellseven under normal oxygen tension. It has been shown [113] that inhibi-tion of glycolysis in tumor cells by three PFK-2 inhibitors, i.e. N-(2-methoxyethyl)-bromoacetamide, N-(2-ethoxyethyl)-bromoacetamide,and N-(3-methoxypropyl)-bromoacetamide, caused anticancer effectsin P388 transplant BDF1 mice and inhibition of tumor cell proliferation.
4.2.1. Regulation of PFK-1 by TP53-induced glycolytic and apoptoticregulator (TIGAR)
Bensaad et al. [33] identified a p53-inducible regulator of glycolysisand apoptosis (TIGAR), which shares functional sequence similaritieswith the bisphosphate domain (FBPase) of PFK-2/FBPase-2 and canmodulate apoptosis in a cell-type-dependent manner. It has been ob-served [115] that cells over-expressing Fru-2,6-bisphosphatase showedenhanced PPP flux and resistance to oxidative stress. Similarly, TIGARcauses decline in Fru-2,6-P2 levels and thereby blocks glycolysis at thisstep, directing the pathway into PPP to produceNADPH and nucleotides.Increasing TIGAR expression, thus, inhibits glycolysis and stimulates PPP.TIGAR can lower ROS levels and decrease sensitivity to p53 and otherROS-associated apoptotic signals, and could be a component in mediat-ing the tumor-suppressing effects of p53 [33]. A model for TIGAR func-tion in anti-oxidative respond has been proposed [33]: i.e. NADPHproduced by PPP is required to generate reduced glutathione (GSH)and thereby decrease ROS levels. GSH, a tripeptide with free sulfhydrylgroup, is required to combat oxidative stress and maintain the normalreduced state in the cells [116]. Oxidized glutathione (GSSG) is reducedto GSH by glutathione reductase using NADPH generated by glucose6-phosphate dehydrogenase (G6PDH), the rate-limiting enzyme of thePPP, and 6-phosphogluconate dehydrogenase. Glutathione peroxidasereduces H2O2 to H2O by oxidizing GSH. Thus, the PPP plays an essen-tial role in protection from oxidative-stress-induced apoptosis [117]and an ability of TIGAR to increase the flux through the PPP leads tothe removal of intracellular ROS. Indeed, treatment of cells withtrans-androsterone, a specific inhibitor of G6PDH, inhibited PPPand prevented the anti-apoptotic activity of TIGAR. Expression ofTIGAR or the isolated FBPase-2 domain increased the GSH/GSSGratio, while decrease of endogenous TIGAR expression lowered it[33].
In addition to regulation of PFK, p53 is also a transcriptional acti-vator of the muscle-specific phosphoglycerate mutase gene and con-tributes in vivo to the control of its cardiac expression [118].
4.2.2. Regulation of PFK-1 by HIF-1αAs mentioned above, the steady state levels of F-2,6-P2 are
maintained by PFK-2/F2,6-Bpase. Minchenko et al. [88] showed thatPFKFB3 was highly induced by hypoxia and that this induction couldbe replicated by the use of an inhibitor of the prolyl hydroxylase

376 J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
enzymes responsible for the von Hippel Lindau (VHL)-dependent de-stabilization and tagging of HIF-1α. The dependence of the PFKFB3gene on HIF-1 was confirmed by its over-expression in VHL-deficientcells and by the lack of hypoxic induction in mouse embryonic fibro-blasts conditionally nullizygous for HIF-1α. Activation of glycolyticgenes byHIF-1 is considered critical formetabolic adaptation to hypoxiathrough increased conversion of glucose to pyruvate and subsequentlyto lactate. Beside PFK-1, HIF-1α is involved in the regulation of almostall the other enzymes in glycolysis [119] (Fig. 1).
4.2.3. Posttranslational modifications of PFK1In human tissues, three types of PFK proteins have been identified:
the muscle type (PFK-M) (MW=85,051Da) [120], liver type (PFK-L)(MW=84,917Da) [121], and type-C (PFK-1, C-type) found in platelets,brain, and other tissues [122]. These isoenzymes are strongly inhibitedby citrate, with IC50 values of 0.08, 0.13 and 0.18mMfor brain (platelet),muscle, and liver PFK1, respectively [123]. PFK1 is normally under thecontrol of feedback inhibition. All human PFK1 isoforms are intenselyinhibited by ATP at concentrations higher than 0.05mM. The negativeeffects of ATP can be antagonized by F-2,6-BP to some extent [124].
A PFK1 isoform less sensitive to citrate inhibition (Ki=0.75mMcitrate) and more sensitive to activation by Fru-2, 6-P2 was seenin human glioma [125]. A PFK1 isoform with similar kinetic char-acteristics was also observed in the fast-growing rodent AS-30Dhepatoma cells, which showed complete insensitivity toward itsallosteric inhibitors, citrate and ATP, in the presence of physiolog-ical concentrations of F-2,6-BP. In addition, the enzyme was highlyactivated by its activators, NH4
+, AMP, and F-2,6-BP [126].A citrate inhibition-resistant form of PFK1 that is activated to a
higher level by allosteric activators has been identified in the fungus[127–129]. These kinetic characteristics are attributed to the 49-kDasubunits, which are relatively small PFK1 molecules with respect toother eukaryotic PFK1s of approximately 85kDa. Further studies haveshown that the shorter 49-kDa fragments are formed by a two-stepposttranslational modification of the native 85-kDa enzyme [128–130].More recently, Smerc et al. [91] reported that the native 85-kDa PFK1,which is normally under the control of feedback inhibition by citrateand ATP, is subjected to posttranslational modification. Proteolyticcleavage of the C-terminal portion of the 85-kDa PFK1 led to an active,shorter 47-kDa fragment that was insensitive to citrate and ATP inhibi-tion. More importantly, only the short 47-kDa fragment but not the na-tive 85-kDa PFK1 was detected in tumorigenic cell lines includingB16-F10 melanoma, Hela cells carcinoma, Nb2-11 lymphoma and Tf-1lymphoma. Similar fragments were also detected in a tumor tissuethat developed in mice after the subcutaneous infection with tumori-genic B16-F10 cells. Limited proteolytic digestion of the rabbit musclePFK-M generated an active citrate inhibition-resistant shorter form, in-dicating that a single posttranslational modification step exists. The in-sertion of modified truncated human pfkM genes also stimulatedglucose consumption and lactate excretion in stable transfectants ofnon-tumorigenic human HEK cell, suggesting the important role ofshorter PFK1 fragments in enhancing glycolytic flux. Thus, posttransla-tional modification of PFK1 enzyme might be the pivotal factor of der-egulated glycolytic flux in tumors that in combination with alteredsignaling mechanisms essentially supports fast proliferation of cancercells. The insertion of modified truncated human pfkM genes also stim-ulated glucose consumption and lactate excretion in stable transfectantsof non-tumorigenic human HEK cell, suggesting the important role ofshorter PFK1 fragments in enhancing glycolytic flux.
4.3. Dysregulation of pyruvate kinase and pyruvate dehydrogenase incancer cells
4.3.1. Dysregulation of pyruvate kinasePyruvate kinase (PK) (EC2.7.1.40) is an enzyme involved in the final
step of glycolysis. It catalyzes the transfer of a phosphate group from
phospoenolpyruvate (PEP) to ADP, yielding one molecule of pyruvateand onemolecule of ATP [131]. In anaerobic glycolysis, lactate dehydro-genase will utilize the NADH produced by glyceraldehyde phosphatedehydrogenase to reduce pyruvate to lactate. PK has four isozymes (L,R, M1, and M2) that are encoded by two different genes of PK L andM. Differential splicing produces L- and R-type PK mRNA and M1- andM2-type PKmRNA from the PK L gene and the PKM gene, respectively.They differ in primary structure and regulation [132].
It have been shown that tumor cells exclusively express the embry-onic M2 isoform of PK (PK-M2) that can be activated by Fru-1,6-P2while binding of tyrosine-phosphorylated peptides to PK-M2 resultsin the release of the allosteric activator, leading to inhibition of enzy-matic activity. PK-M2 has been known to play an important role in an-aerobic glycolysis and cancer metabolism [133]. Deactivation of PK-M2in tumor cells is believed to divert glucosemetabolism from energy pro-duction to anabolic processes [133,134].
PK-M2 is important for the growth of several human cell lines in-cluding glioma cell lines, brain tumor stem cells, and transformedhuman astrocytes [135]. Decreased expression and activity of PK-M2have been linked to cisplatin resistance in human gastric carcinomacell lines [136] and oxaliplatin resistance in patientswith colorectal can-cer and in human cell lines [137]. PK-M2 has been identified as a directtarget of the tumor-suppressive miR-326. Glioma cells with high levelsof PK-M2 expressed lower levels of miR-326, suggestive of endogenousregulation of PKM2 bymiR-326. It is also expressed in primary glioblas-toma samples but absent in normal brain temporal lobe. siRNA knock-down of PK-M2 leads to decreased glioma cell and glioma stem cellproliferation, invasiveness, clonogenicity, and survival. Furthermore,PK-M2 knockdown impaired glioma cell metabolism, with decreasedglutathione and ATP levels and increased activation of AMP-activatedprotein kinase. Since PK-M2 is expressed in glioma cells and is impor-tant for their survival, but is lacking in the normal brain, its manipula-tion may serve as a brain-sparing therapy for glioblastoma [135].
Two missense mutations, H391Y and K422R, in PK-M2 have beenfound in cells from Bloom syndrome patients, prone to develop can-cer. The H391Y showed a 6-fold increase in affinity for its substratePEP and behaved like a non-allosteric protein with compromised co-operative binding, whereas the affinity for PEP was lost significantlyin K422R. Unlike K422R, H391Y showed enhanced thermal stability,stability over a range of pH values, a lesser effect of the allosteric in-hibitor and resistance toward structural alteration upon binding ofthe activator (Fro-1,6-BP) and inhibitor Both mutants showed aslight shift in the pH optimum from 7.4 to 7.0 [138]. Gupta et al.[139] further investigated the mechanisms and functional implica-tion of the H391Y and K422R mutations of PKM2. They observed thatthe co-expression of homotetrameric wild type and mutant PKM2 in thecellular milieu resulted in substantiated interaction between the two atthe monomer level in vitro experiments and that the cross-monomer in-teraction significantly altered the oligomeric state of PK-M2 by favoringdimerization and heterotetramerization. In silico study provided anadded support in showing that hetero-oligomerization was energeticallyfavorable. The hetero-oligomeric populations of PK-M2 showed alteredactivity and affinity, and their expression resulted in an increased growthrate ofmammalian cells, alongwith an increased rate of polyploidy. Thesefeatures are known to be essential to tumor progression.
4.3.2. Regulation of pyruvate dehydrogenaseThe pyruvate dehydrogenase (PDH) complex contributes to trans-
forming pyruvate into acetyl-CoA via pyruvate decarboxylation.Acetyl-CoA may then be used in the TCA cycle to carry out cellularrespiration. Thus, PDH contributes to linking the glycolysis metabolicpathway to the TCA cycle and releasing energy via NADH.
Pyruvate dehydrogenase kinase (PDK) (EC 2.7.11.2) is a kinase en-zyme which acts to inactivate the enzyme activity of PDH byphosphorylation-dephosphorylation of three specific serine residues(site 1, Ser-264; site 2, Ser-271; site 3, Ser-203) of the α subunit of

377J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
the pyruvate dehydrogenase (E1) component in PDH using ATP [140].Normally, the active site of PDH is in a stabilized and ordered confor-mation supported by a network of hydrogen bonds. Phosphorylationat site 1 by PDK causes steric clashes with another nearby serine res-idue due to both the increased size and negative charges associatedwith the phosphorylated residue [141]. This disrupts the hydrogenbond network and disorders the conformation of two phosphoryla-tion loops. These loops prevent the reductive acetylation step, thushalting overall activity of the enzyme [142]. By downregulating theactivity of this complex, PDK decreases the oxidation of pyruvate inmitochondria and increases the conversion of pyruvate to lactate inthe cytosol.
Like PFK, PDK is regulated both by allosteric effectors and by co-valent modifications such as phosphorylation. PDK is stimulated byATP, NADH and acetyl-CoA but is inhibited by ADP, NAD+, CoA-SH,pyruvate, and alanine (a biosynthetic product of pyruvate) actingas allosteric inhibitors of PDK [143]. The activity of PDK has beenshown to be consistently higher in cancer cells than in normal cells[144].
There are four known isozymes of PDK, namely PDK1, PDK2, PDK3and PDK4, in humans and they share 70% identity but differ greatlynear their N-terminus [145]. PDK1 is ample in heart cells. PDK3 ismost abundant in testis. PDK2 is present in most tissues but low inspleen and lung cells. PDK4 is predominantly found in skeletal muscleand heart tissues [140].
It has been shown that HIF-1 is involved in regulation of PDK1[146,147]. PDK1 has shown to have increased activity in hypoxic cancercells due to the presence of HIF-1. Kim et al. [146] observed that HIF-1αsuppresses metabolism through TCA cycle by directly trans-activatingthe PDK1. Forced PDK1 expression in hypoxia HIF-1α null cells in-creases ATP levels, attenuates hypoxic ROS generation, and rescuesthese cells from hypoxia-induced apoptosis. These studies reveal ahypoxia-induced metabolic switch that shunts glucose metabolismsfrom the mitochondria to glycolysis to maintain ATP production andto prevent toxic ROS production.
Although hypoxic intratumoral conditions account for HIF-1αstabilization and induction of anaerobic metabolism, recent studiessuggest that high pyruvate concentrations also result in HIF1α stabi-lization independently of hypoxia. Koukourakis et al. [147] observedthat the PDH/PDK pathway is repressed in 73% of non-small cell lungcarcinomas, which may be a key reason for HIF-1α stabilization and“aerobic glycolysis.” However, about half of PDH-deficient carcino-mas are unable to switch on the HIF-1 pathway, and patients harbor-ing these tumors have an excellent postoperative outcome. A smallsubgroup of clinically aggressive tumors maintains a coherent PDHand HIF/LDH5 expression. In contrast to cancer cells, fibroblasts inthe tumor-supporting stroma exhibit an intense PDH but reducedPDK1 expression favoring maximum PDH activity. This means thatstroma may use lactic acid produced by tumor cells, preventing thecreation of an intolerable intratumoral acidic environment at thesame time.
PDK1 inhibition has been suggested as an antitumor therapy sincePDK1 prevents apoptosis in these cancerous cells [148]. Indeed,AZD7545 and dichloroacetate have been shown to bind to PDK1 andinhibit its kinase activity [149]. These inhibitors have the similar in-hibitory effects on PDK3 [149], which are overexpressed in colon can-cer cell lines [150]. An inhibitor of PK-M2, dichloroacetic acid (DCA),can block growth of tumors in xenograft models [34].
More recently, Contractor et al. [34] reported that p53 nega-tively regulated PDK transcription by decreasing levels of PDK-2and its product, the inactive form of the PDH complex (P-Pdc),both of which are key regulators of pyruvate metabolism. De-creased levels of PDK2 and P-Pdc in turn promoted conversionof pyruvate into acetyl-CoA instead of lactate. Thus, wild-typep53 limited lactate production in cancer cells unless Pdk2 couldbe elevated.
5. Altered expression/functions of enzymes/tumor suppressorsinvolved TCA cycle
5.1. TCA cycle in cancer cells
TCA cycle (also known as citric acid cycle or the Krebs cycle)(Fig. 2) is a series of chemical reactions used by all aerobic living or-ganisms to generate energy through the oxidization of pyruvate de-rived from carbohydrates, fats and proteins into carbon dioxide andwater. In addition, TCA cycle provides precursors for the biosynthesisof compounds including certain amino acids as well as the reducingagent NADH that is used in numerous biochemical reactions. It is cen-tral importance for many biochemical pathways. Because of its crucialrole for basal metabolism of the cells, TCA cycle has long been consid-ered as no significant and primary defect in its enzyme components.However, genetic studies have revealed that mutations in several mi-tochondrial proteins including succinate dehydrogenase (SDH) andfumarate hydratase (FH), which lead to dysfunction of TCA cycle,could be a cause of human diseases and tumor formation (for reviewsee [16]).
5.2. Mutations of genes that encode enzymes in TCA cycle in cancer cells
Several mitochondrial proteins including SDH and FH, both are en-zymes of TCA cycle, are tumor suppressors. It has been shown that anumber mutations in genes that encode enzymes including aconitase,isocitrate dehydrogenase 1(IDH1), SDH and FH in TCA cycle lead tosome types of cancer.
5.2.1. Mitochondrial aconitase in cancer cellsAconitase is a mitochondrial enzyme that catalyzes the stereo-
specific isomerization of citrate to isocitrate via cis-aconitate in theTCA cycle [151,152]. Aconitase is regarded as the key enzyme in cit-rate oxidation in human prostate epithelial cells, a unique organthat produces and releases large amounts of citrate. The abnormal ex-pression and activity of aconitase have been implicated in tumorigen-esis of the prostate [153]. It has been observed that the mRNA levelsof mitochondrial and cytosolic aconitases and aconitase activity aswell as fatty acid synthase are significantly higher in metastaticPC-3M cells than in normal human prostate cells (PNT2-C2) [153].Using immunohistochemical analysis of prostate cancer tissuesections and malignant prostate cell lines, Singh et al. [154]detected the presence of mitochondrial-aconitase (m-aconitase) inthe mitochondrial compartment in PC-3, LNCaP, and DU-145 malig-nant prostate cell lines and prostate tissue sections from prostatecancer subjects where mitochondrial aconitase enzyme is presentin the glandular epithelium of normal glands, hyperplastic glands,adenocarcinomatous glands, and prostatic intraepithelial neoplasticfoci. However, no significant difference in m-aconitase enzymelevels was seen in the glandular epithelium of citrate-producing ad-enomatous glands versus the citrate-oxidizing adenocarcinomatousglands. Tsui et al. [155] observed that p53 down-regulated the geneexpression of m-aconitase in human prostate carcinoma cells.
5.2.2. Mutations of IDH1 in cancer cellsIDH1 converts isocitrate to α-ketoglutamate (2-KG) with genera-
tion of NADH. Up to 12% of glioblastoma tumors have spontaneouspoint mutations in IDH1 genes [156]. Mutations that affected aminoacid132 of IDH1 have also been identified in grades II and III astrocyto-mas, oligodendrogliomas and glioblastomas that developed fromthese lower-grade lesions [157]. Similar mutations in IDH2 at residueArg172 in the active site have been identified in acute myeloid leuke-mia [158] and other diseases [159]. These mutations disable theenzyme's normal ability to convert isocitrate to 2-KG and confer onthe enzymes a new function, i.e. the ability to convert 2-KG tod-2-hydroxyglutarate (D-2-HG) [159,160]. The elevated levels of

Fig. 2. Regulation of TCA cycle and glutaminolysis by C-Myc, HIF-1 and p53.
378 J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
2HG in vivo are thought to contribute to the formation and malignantprogression of gliomas [160].
5.2.3. Mutations of SDHD in cancer cellsSDHD, an enzyme complex consisting of four subunits A, B, C and D,
catalyzes the oxidation of succinate to fumarate with the reduction ofubiquinone to ubiquinol. SDHD is bound to the innermembrane ofmam-malian mitochondria. It is the only enzyme that participates in both theTCA cycle and the electron transport chain [161]. Inherited or somaticmutations in subunits B, C, or D of SDH have been associatedwith severaltypes of cancers including pheochromocytoma, paraganglioma renal cellcarcinoma and papillary thyroid cancers [162–166]. Reduced expressionand loss of heterozygosity of SDHD gene are observed in gastric andcolon carcinoma [167]. The R22X mutation of SDHD gene in hereditaryparaganglioma abolishes the enzymatic activity of complex II of mito-chondrial respiratory chain and activates HIF-1α, leading to increasedexpression of HIF-1α-regulated genes [168–170]. Selak et al. [171] haveshown that succinate is accumulated due to SDHD down-regulationand transmits an “oncogenic” signal from mitochondria to the cytosol.Once in the cytosol, succinate inhibits HIF-1α prolyl hydroxylase, leadingto HIF-1α stabilization under normoxic conditions. Thus, succinate canincrease expression of genes that facilitate angiogenesis, metastasis,and glycolysis, ultimately leading to tumor progression. These resultssuggest a mechanistic link between SDHDmutations and HIF-1α induc-tion, providing an explanation for the highly vascular tumors that devel-op in the absence of VHL mutations.
5.2.4. Mutations of FU in cancer cellsFH is an enzyme of the TCA cycle that catalyzes the reversible hy-
dration/dehydration of fumarate to malate. Mutations in the FH genehave been identified in chromosome 1q42.3-43 mapped to the genet-ic locus for multiple cutaneous and uterine leiomyoma syndrome
(MCL), which is inherited in an autosomal dominant pattern, man-ifesting as skin leiomyoma and uterine fibroids in affected individuals[172]. Germline mutations in the FH gene predispose to multiple MCLand MCL-associated renal cell cancer. Martinez-Mir et al. [173] per-formed the clinical and mutational analysis of five families withMCL. They identified five mutations that affect the highly conservedresidues of the FH protein. These mutations include a nonsense muta-tion Q142X (amino acid residue 185 in the mitochondrial isoform); adeletion of four nucleotides in exon 7 and three missense mutations,i.e. a G>T transversion at nucleotide position 473 in exon 4, leading tothe missense mutation S115I (mitochondrial amino acid residue 158;family MCL-4), a C>T transition at position 952 in exon 7 resulting inthe missense mutation H275Y (mitochondrial amino acid residue 318;family MCL-1), and a G>C transversion at position 1180 in exon 8, cre-ating the missense mutation V351L (mitochondrial amino acid residue394; family MCL-3). These results provide evidence for the role of theFH gene in the pathogenesis of MCL. Reduced FH expression has beenseen in clear cell renal cancer, the most common histologic variant ofkidney cancer, leading to the accumulation of HIF-2α, which is knownto promote renal carcinogenesis, migration and invasion whereasover-expression of FH in renal cancer cells inhibits cellular migrationand invasion [174]. These data provide novel insights into the tumorsuppressor functions of FH in sporadic kidney cancer.
FH-deficient cells and tissues accumulate high levels of fumarate,which may act as an oncometabolite and contribute to tumorigenesis.Fumarate has been proposed to have a role in the covalent modificationof cysteine residues to S-(2-succinyl) cysteine (2SC) (termed proteinsuccination). Bardella et al. [175] assessed 2SC levels in the models ofhereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome.They observed robust detection of 2SC in Fh1 (murine FH)-deficientrenal cysts and in a retrospective series of HLRCC tumors withestablished FHmutations. Importantly, 2SCwas undetectable in normal

379J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
tissues and tumor types not associated with HLRCC. In a prospectiveevaluation of cases referred for genetic testing for HLRCC, the presenceof 2SC-modified proteins (2SCP) correctly predicted genetic alterationsin FH in every case. In two series of unselected type II papillary renalcancer (PRCC), prospectively analyzed by 2SCP staining followed by ge-netic analysis, the biomarker accurately identified FH mutations. Morerecent studies [69,176] have revealed that fumarate modifies cysteineresidues within the Kelch-like ECH-associated protein 1 (KEAP1),which inhibits the activity of nuclear factor (erythroid-derived 2)-like2 (Nrf2). Nrf2 has the ability to achieve cytoprotection by regulatingthe expression of antioxidative stress enzymes/proteins. Thus, this2SCP modification in KEAP1 abrogates its ability to repress theNrf2-mediated antioxidant response pathway. These studies suggest arole for KEAP1/Nrf2 dysregulation in FH-associated tumors.
6. Regulation of glutaminolysis by C-Myc and p53
6.1. Glutamine and glutaminolysis
Glutamine is the most abundant free amino acid in the circulationand in intracellular pools. It is not only a precursor for the synthesis ofamino acids, proteins, nucleotides, and a number of biologically impor-tant molecules, but also play a regulatory role in several cell specificprocesses includingmetabolism (e.g. oxidative fuel, gluconeogenic pre-cursor, and lipogenic precursor), cell integrity (apoptosis and cell prolif-eration), protein synthesis, and degradation, contractile protein mass,redox potential, respiratory burst, insulin resistance, insulin secretion,and extracellularmatrix (ECM) synthesis. Glutamine, an essential nutri-ent for cancer cell proliferation, has been shown to regulate the expres-sion of many genes related to metabolism, signal transduction, celldefense and repair, and to activate intracellular signaling pathways.Thus, the function of glutamine goes beyond that of a simple metabolicfuel or protein precursor [177–180].
Glutaminolysis is a series of biochemical reactions by which gluta-mine is degraded to glutamate, aspartate, CO2, pyruvate, lactate, ala-nine and citrate. Glutamine is imported into cells through highaffinity surface glutamine importers such as ASCT2 and SN2 [18].Once it enters the cells, the majority of glutamine either donates ni-trogen to macromolecules or is deamidated by glutaminases, whichconverts glutamine to glutamate (Glu). Glu has several fates. It canbe converted directly to glutathione (GSH) by glutathione cysteine li-gase (GCL). The reduced GSH, one of the most abundant anti-oxidantspresent in mammalian cells, is vital to controlling the redox state ofthe subcellular compartments [181]. Glu can also be catabolizedthrough removal of α-nitrogen by aminotransferases or glutamatedehydrogenase (GDH) produces α-KG, an integral component ofTCA cycle through the TCA cycle for the production of ATP or servesas substrate for biosynthesis of the polyglutamated folic acid orprocessed further in mitochondria. The cyclization of glutamate pro-duces proline, an amino acid important for synthesis of collagen andconnective tissue. Alternatively, some tissues e.g. rat liver andbrown adipocyte cell lines can reductively carboxylate α-KG to gen-erate citrate [182,183]. There is evidence indicating that a fractionglutamine-derived carbon can exit the TCA cycle as malate andserve as substrate of malic enzymes 1, which produces NADPH[184] More recently, Metallo et al. [185] have shown that human cellsuse reductive metabolism of α-KG to synthesize acetyl-CoA for lipidsynthesis. This IDH1-dependent pathway is active in most cell linesunder normal culture conditions, but cells grown under hypoxia rely al-most exclusively on the reductive carboxylation of glutamine-derivedα-KG for de novo lipogenesis. Furthermore, renal cell lines deficient inthe von Hippel–Lindau tumor suppressor protein preferentially use re-ductive glutaminemetabolism for lipid biosynthesis even at normal ox-ygen levels. These results identify a critical role for oxygen in regulatingcarbon use to produce actyl-CoA and support lipid synthesis in mam-malian cells. It has been reported [186] that 17β-estradiol increased
the carbonflow through PPP and enhanced glutamine consumption pri-marily to provide biosynthetic precursor.
6.1.1. Regulation of glutamine transport and glutaminase-1 by c-MycIn order to compensate for “Warburg's Effect” and to help main-
tain a functioning TCA cycle, cancer cells often rely on elevated gluta-mine metabolism through a marked elevation of glutaminase activity[187]. Several studies [18,19,43] have revealed that c-Myc plays amajor role in regulating glutaminolysis.
Le et al. [43] investigated the metabolic responses of a c-Myc-inducible human Burkitt lymphoma model P493 cell line to aerobicandhypoxic conditions, and to glucose deprivation, using [U-13C]-glucoseas the tracer isotope-for resolvingmetabolomics. They observed that glu-tamine import and metabolism through the TCA cycle persisted underhypoxia, and that glutamine contributed significantly to citrate carbons.Under glucose deprivation, glutamine-derived fumarate, malate, and cit-ratewere significantly increased. The 13C-labeling patterns demonstratedan alternative energy-generating glutaminolysis pathway involving aglucose-independent TCA cycle.
There are three isoforms of glutaminases, GLS1, GLS2 and GLSC(a splice variant of GLS1) [180]. GLS1 is required for cell cycle pro-gression through S phase to cell division [188]. GLS2 gene encodesa mitochondrial glutaminase, a key enzyme that catalyzes the hy-drolysis of glutamine to glutamate and thereby a regulator of gluta-thione (GSH) synthesis and energy production [189,190]. It hasbeen shown that glutamine importers and GLS1 expression areup-regulated by c-Myc [18,19,191] and that GLS2 expression isup-regulated by p53 [35,36,192].
Wise et al. [18] reported that c-Myc activated the transcription ofglutamine importers ASCT2 and SN2 by selectively binding to the pro-moter regions of both genes that are required for glutamine uptake andmetabolism. A consequence of this c-Myc-dependent glutaminolysis isthe reprogramming of mitochondrial metabolism to depend on gluta-mine catabolism to sustain cellular viability and TCA cycle anaplerosis.The ability of c-Myc-expressing cells to engage in glutaminolysis doesnot depend on concomitant activation of PI3K or AKT. The stimulationof mitochondrial glutamine metabolism resulted in reduced glucosecarbon entering the TCA cycle and a decreased contribution of glucoseto the mitochondrial-dependent synthesis of phospholipids. c-Myc hasbeen found to induce glutamine transporters SLC5A1 and SLC7A1 andthus directly increase glutamine uptake [6,19]. Gao et al. [19] reportedthat the c-Myc transcriptionally up-regulated the expression of mito-chondrial glutaminase-1 in human P-493 B lymphoma cells and PC3prostate cancer cells through repressing microRNAs miR-23a andmiR-23b that target the GLS's 3′ untranslating regions and that gluta-mine and glutaminase are necessary for Myc-mediated cancer cell pro-liferation and survival. The unique means by which c-Myc regulatesglutaminase uncovers a link between c-Myc regulation of miRNAs, glu-tamine metabolism, and ROS homeostasis. Together, these studies sug-gest that oncogenic levels of c-Myc induce a transcriptional programthat promotes glutaminolysis and triggers cellular addiction to gluta-mine as a bioenergetic substrate.
6.2. Regulation of GLS2 by p53 and GLS2 as an potential target forcancer therapy
GLS2 regulates cellular energy metabolism by increasing produc-tion of glutamate and α-KG, which in turn results in enhanced mito-chondrial respiration and ATP generation. Furthermore, GLS2 alsoregulates antioxidant defense function in cells by increasing reducedglutathione (GSH) levels and decreasing ROS levels, which, in turn,protects cells from oxidative stress-induced apoptosis. It has beenshown that activation of p53 increases the GLS2 expression underboth non-stressed and stressed conditions and increases the levelsof glutamate and α-KG, mitochondrial respiration rate, and GSHlevels, and decreases ROS levels in cells [35,36].

380 J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
GSL2 has been identified as a unique p53 target gene to mediate therole of p53 in both cellular energy metabolism and antioxidant defensemechanism, as demonstrated by several lines of evidence [35,36,122]:a) human GLS2 gene promoter contains a p53 consensus DNA-bindingelement and its expression is induced in response toDNAdamage or ox-idative stress in a p53-dependent manner and p53 associates with theGLS2 promoter; b) p53 and GLS2 modulate intracellular ROS levelsand GSH/GSSG ratio in the cells and elevated GLS2 levels facilitate glu-tamine metabolism and lowers intracellular ROS levels, resulting in anoverall decrease in DNA oxidation, and GLS2 protect cells from DNA ox-idation and ROS-sensitive apoptosis; c) the expression of GLS2 is loss inhuman liver tumors whereas GLS2 over-expression reduces tumor cellcolony formation abilities. Furthermore, GLS2 expression is reduced inliver tumors compared with normal tissues. These results demonstratethat GLS2, as a unique p53 target gene, is a mediator of p53 for its rolesin energymetabolism and antioxidant defense, which can contribute toits role in tumor suppression and repairable genotoxic stress
The essential role of glutamine metabolism in cell survival andproliferation under hypoxia and glucose deficiency makes these can-cer cells susceptible to the glutaminase inhibitor BPTES and hencecould be targeted for cancer therapy. For example, Wang et al. [193]identified a small molecule inhibitor that blocks oncogenic transfor-mation induced by Rho GTPases in fibroblasts, and the growth ofhuman breast cancer and B lymphoma cells, without affecting normalcells. The target of this inhibitor has been identified to be glutamin-ase. Transformed fibroblasts and breast cancer cells have been observedto exhibit elevated glutaminase activity, which is dependent on RhoGTPases and NF-κB activity and is blocked by the small molecule inhib-itor. These findings demonstrate that targeting glutaminase activity caninhibit oncogenic transformation. Furthermore, a glutamine-analog,acivicin, has been found to inhibit tumor growth and tumor-inducedangiogenesis in Ehrlich ascites carcinoma. Toy et al. [194] reportedthat combination of acivicin with E. coli glutaminase synergistically re-duced in vitro proliferation and matrigel invasion of human MCF-7and OAW-42 cells through inhibiting the release of VEGF and MMP-9by cells in culture supernatant significantly than single agent treatment.
7. Conclusion remarks
In this work, we described the current knowledge about the dys-regulation of the key proteins/enzymes involved in glucose transport,glycolysis, TCA cycle and glutaminolysis by p53, c-Myc and HIF-1α incancer cells, as summarized in Figs. 1 and 2. The tumor suppressorp53 is involved in negative regulation of the key enzymes/protein fac-tors including GTUTs 1–4 in glucose transport, HK, PFK, phosphoglyc-erate mutase, and PDK in glycolysis, and aconitase in TCA cycle. P53also acts as positive regulator of GLS2 in glutaminolysis. Because ho-mozygous loss and mutations of p53 are frequently found in manytypes of cancers [195], p53 could lost its regulatory functions in con-trolling these energy metabolism pathways, leading to abnormal ex-pression of the enzymes/proteins involved in the key steps of thesemetabolic pathways.
The oncogenes C-Myc and HIF-1 contribute to the pathogenesis ofmany human cancers through linking altered cellular metabolism totumorigenesis. The c-Myc regulates the expression of GLUTs1–4 inglucose transport. The c-Myc and HIF-1 are involved in the regulationof almost of all the enzymes in glycolyis pathway [5,6]. The c-Myc alsodirectly regulates the glutamine transporters, ASCT2 and SN2, and/oris indirectly involved in enhancing glutaminase protein expressionand glutamine metabolism by repression of microRNA 2, 3a/b. In ad-dition to playing an important role in regulation of almost all the gly-colytic enzymes [5], HIF-1 is involved in regulation of TCA cycle bybinding to succinate Co-A synthase.
The alterations to cellular metabolism in cancer cells have been in-creasingly recognized as a crucial hallmark of cancer [7,196]. The alter-ations in the key steps in glucose transport and energy metabolism
pathways by abnormal functions of c-Myc, HIF-1 and the loss functionof p53 and perhaps other oncogenes and tumor suppressors in cancercells have been used as biomarkers for diagnostic tools. Indeed, it wasrecently reported that up regulation of GLUT-1, HK-I, and HIF-1α wasclosely related with 18F-FDG uptake into mesothelioma cells, and thatmTOR inhibitor induced a decrease in GLUT-1 expression and 18F-FDGuptake [197,198]. Furthermore, these important biomarkers/targetshave been proposed to be translated for the development of promisinganticancer therapies [5,6,40].
Acknowledgements
JQC would like to devote this manuscript to the late professorsShuping Weng and Mingqi Li of South China Agricultural University(SCAU) and to the late professor Clanton C Black Jr. of University ofGeorgia (UGA), the major professors who introduced and led JQCinto the fields of photosynthesis, photorespiration, carbohydrate/en-ergy metabolism pathways and glutaminolysis in plants during hisMS and Ph.D. graduate studies at SCAU and UGA, respectively.
References
[1] O. Warburg, On the origin of cancer cells, Science 123 (1956) 309–314.[2] W.H. Koppenol, P.L. Bounds, C.V. Dang, Otto Warburg's contributions to current
concepts of cancer metabolism, Nat. Rev. Cancer 11 (2011) 325–337.[3] R.G. Jones, C.B. Thompson, Tumor suppressors and cell metabolism: a recipe for
cancer growth, Genes Dev. 23 (2009) 537–548.[4] K. Garber, Energy deregulation: licensing tumors to grow, Science 312 (2006)
1158–1159.[5] S.J. Yeung, J. Pan, M.H. Lee, Roles of p53, MYC and HIF-1 in regulating glycolysis—
the seventh hallmark of cancer, Cell. Mol. Life Sci. 65 (2008) 3981–3999.[6] R.A. Cairns, I.S. Harris, T.W. Mak, Regulation of cancer cell metabolism, Nat. Rev.
Cancer 11 (2011) 85–95.[7] R.J. DeBerardinis, J.J. Lum, G. Hatzivassiliou, C.B. Thompson, The biology of can-
cer: metabolic reprogramming fuels cell growth and proliferation, Cell Metab.7 (2008) 11–20.
[8] H.L. Wieman, J.A. Wofford, J.C. Rathmell, Cytokine stimulation promotes glucoseuptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity andtrafficking, Mol. Biol. Cell 18 (2007) 1437–1446.
[9] A.L. Edinger, C.B. Thompson, Akt maintains cell size and survival by increasingmTOR-dependent nutrient uptake, Mol. Biol. Cell 13 (2002) 2276–2288.
[10] L.E. Huang, Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation,Cell Death Differ. 15 (2008) 672–677.
[11] C.V. Dang, Rethinking the Warburg effect with Myc micromanaging glutaminemetabolism, Cancer Res. 70 (2010) 859–862.
[12] N. Palaskas, S.M. Larson, N. Schultz, E. Komisopoulou, J. Wong, D. Rohle, C. Campos,N. Yannuzzi, J.R. Osborne, I. Linkov, E.R. Kastenhuber, R. Taschereau, S.B. Plaisier, C.Tran, A. Heguy, H. Wu, C. Sander, M.E. Phelps, C. Brennan, E. Port, J.T. Huse, T.G.Graeber, I.K. Mellinghoff, 18F-fluorodeoxy-glucose positron emission tomographymarks MYC-overexpressing human basal-like breast cancers, Cancer Res. 71(2011) 5164–5174.
[13] G.L. Semenza, HIF-1: upstream and downstream of cancer metabolism, Curr.Opin. Genet. Dev. 20 (2010) 51–56.
[14] F. Weinberg, R. Hamanaka, W.W. Wheaton, S. Weinberg, J. Joseph, M. Lopez, B.Kalyanaraman, G.M. Mutlu, G.R. Budinger, N.S. Chandel, Mitochondrial metabo-lism and ROS generation are essential for Kras-mediated tumorigenicity, Proc.Natl. Acad. Sci. U. S. A. 107 (2010) 8788–8793.
[15] A.J. Levine, A.M. Puzio-Kuter, The control of the metabolic switch in cancers byoncogenes and tumor suppressor genes, Science 330 (2010) 1340–1344.
[16] J.J. Briere, J. Favier, A.P. Gimenez-Roqueplo, P. Rustin, Tricarboxylic acid cycledysfunction as a cause of human diseases and tumor formation, Am. J. Physiol.Cell Physiol. 291 (2006) C1114–C1120.
[17] F. Li, Y. Wang, K.I. Zeller, J.J. Potter, D.R. Wonsey, K.A. O'Donnell, J.W. Kim, J.T.Yustein, L.A. Lee, C.V. Dang, Myc stimulates nuclearly encoded mitochondrialgenes and mitochondrial biogenesis, Mol. Cell. Biol. 25 (2005) 6225–6234.
[18] D.R. Wise, R.J. DeBerardinis, A. Mancuso, N. Sayed, X.Y. Zhang, H.K. Pfeiffer, I.Nissim, E. Daikhin, M. Yudkoff, S.B. McMahon, C.B. Thompson, Myc regulatesa transcriptional program that stimulates mitochondrial glutaminolysis and leadsto glutamine addiction, Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 18782–18787.
[19] P. Gao, I. Tchernyshyov, T.C. Chang, Y.S. Lee, K. Kita, T. Ochi, K.I. Zeller, A.M. DeMarzo, J.E. Van Eyk, J.T. Mendell, C.V. Dang, c-Myc suppression of miR-23a/benhances mitochondrial glutaminase expression and glutamine metabolism,Nature 458 (2009) 762–765.
[20] E.I. Chen, J. Hewel, J.S. Krueger, C. Tiraby, M.R. Weber, A. Kralli, K. Becker, J.R.Yates III, B. Felding-Habermann, Adaptation of energy metabolism in breast can-cer brain metastases, Cancer Res. 67 (2007) 1472–1486.
[21] X. Lu, B. Bennet, E. Mu, J. Rabinowitz, Y. Kang, Metabolomic changes accompany-ing transformation and acquisition of metastatic potential in a syngeneic mousemammary tumor model, J. Biol. Chem. 285 (2010) 9317–9321.

381J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
[22] A.D. Richardson, C. Yang, A. Osterman, J.W. Smith, Central carbon metabolism inthe progression of mammary carcinoma, Breast Cancer Res. Treat. 110 (2008)297–307.
[23] R.J. Deberardinis, N. Sayed, D. Ditsworth, C.B. Thompson, Brick by brick: metab-olism and tumor cell growth, Curr. Opin. Genet. Dev. 18 (2008) 54–61.
[24] G. Kroemer, J. Pouyssegur, Tumor cell metabolism: cancer's Achilles' heel, Can-cer Cell 13 (2008) 472–482.
[25] B. Vogelstein, D. Lane, A.J. Levine, Surfing the p53 network, Nature 408 (2000)307–310.
[26] S. Sengupta, C.C. Harris, p53: traffic cop at the crossroads of DNA repair and re-combination, Nat. Rev. Mol. Cell Biol. 6 (2005) 44–55.
[27] K. Polyak, Y. Xia, J.L. Zweier, K.W. Kinzler, B. Vogelstein, A model for p53-inducedapoptosis, Nature 389 (1997) 300–305.
[28] T. Waldman, K.W. Kinzler, B. Vogelstein, p21 is necessary for the p53-mediatedG1 arrest in human cancer cells, Cancer Res. 55 (1995) 5187–5190.
[29] S. Macip, M. Igarashi, P. Berggren, J. Yu, S.W. Lee, S.A. Aaronson, Influence of in-duced reactive oxygen species in p53-mediated cell fate decisions, Mol. Cell.Biol. 23 (2003) 8576–8585.
[30] K. Chen, A. Albano, A. Ho, J.F. Keaney Jr., Activation of p53 by oxidative stress in-volves platelet-derived growth factor-beta receptor-mediated ataxia telangiectasiamutated (ATM) kinase activation, J. Biol. Chem. 278 (2003) 39527–39533.
[31] A.A. Sablina, A.V. Budanov, G.V. Ilyinskaya, L.S. Agapova, J.E. Kravchenko, P.M.Chumakov, The antioxidant function of the p53 tumor suppressor, Nat. Med.11 (2005) 1306–1313.
[32] F. Schwartzenberg-Bar-Yoseph, M. Armoni, E. Karnieli, The tumor suppressorp53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression,Cancer Res. 64 (2004) 2627–2633.
[33] K. Bensaad, A. Tsuruta, M.A. Selak, M.N. Vidal, K. Nakano, R. Bartrons, E. Gottlieb,K.H. Vousden, TIGAR, a p53-inducible regulator of glycolysis and apoptosis, Cell126 (2006) 107–120.
[34] T. Contractor, C.R. Harris, p53 negatively regulates transcription of the pyruvatedehydrogenase kinase Pdk2, Cancer Res. 72 (2012) 560–567.
[35] W. Hu, C. Zhang, R. Wu, Y. Sun, A. Levine, Z. Feng, Glutaminase 2, a novel p53 tar-get gene regulating energy metabolism and antioxidant function, Proc. Natl.Acad. Sci. U. S. A. 107 (2010) 7455–7460.
[36] S. Suzuki, T. Tanaka, M.V. Poyurovsky, H. Nagano, T. Mayama, S. Ohkubo, M.Lokshin, H. Hosokawa, T. Nakayama, Y. Suzuki, S. Sugano, E. Sato, T. Nagao, K.Yokote, I. Tatsuno, C. Prives, Phosphate-activated glutaminase (GLS2), ap53-inducible regulator of glutamine metabolism and reactive oxygen species,Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 7461–7466.
[37] S. Matoba, J.G. Kang, W.D. Patino, A. Wragg, M. Boehm, O. Gavrilova, P.J. Hurley,F. Bunz, P.M. Hwang, p53 regulates mitochondrial respiration, Science 312(2006) 1650–1653.
[38] P. Jiang, W. Du, X. Wang, A. Mancuso, X. Gao, M. Wu, X. Yang, p53 regulates bio-synthesis through direct inactivation of glucose-6-phosphate dehydrogenase,Nat. Cell Biol. 13 (2011) 310–316.
[39] E. Gottlieb, p53 guards the metabolic pathway less travelled, Nat. Cell Biol. 13(2011) 195–197.
[40] C.V. Dang, A. Le, P. Gao, MYC-induced cancer cell energy metabolism and thera-peutic opportunities, Clin. Cancer Res. 15 (2009) 6479–6483.
[41] A. Frenzel, J. Loven, M.A. Henriksson, Targeting MYC-regulated miRNAs to com-bat cancer, Genes Cancer 1 (2010) 660–667.
[42] C.V. Dang, MYC on the path to cancer, Cell 149 (2012) 22–35.[43] A. Le, A.N. Lane, M. Hamaker, S. Bose, A. Gouw, J. Barbi, T. Tsukamoto, C.J. Rojas,
B.S. Slusher, H. Zhang, L.J. Zimmerman, D.C. Liebler, R.J. Slebos, P.K. Lorkiewicz,R.M. Higashi, T.W. Fan, C.V. Dang, Glucose-independent glutamine metabolismvia TCA cycling for proliferation and survival in B cells, Cell Metab. 15 (2012)110–121.
[44] D.M. Brizel, G.L. Rosner, L.R. Prosnitz, M.W. Dewhirst, Patterns and variability oftumor oxygenation in human soft tissue sarcomas, cervical carcinomas, andlymph node metastases, Int. J. Radiat. Oncol. Biol. Phys. 32 (1995) 1121–1125.
[45] G.L. Semenza, Expression of hypoxia-inducible factor 1: mechanisms and conse-quences, Biochem. Pharmacol. 59 (2000) 47–53.
[46] M. Hayashi, M. Sakata, T. Takeda, T. Yamamoto, Y. Okamoto, K. Sawada, A.Kimura, R. Minekawa, M. Tahara, K. Tasaka, Y. Murata, Induction of glucosetransporter 1 expression through hypoxia-inducible factor 1alpha under hypox-ic conditions in trophoblast-derived cells, J. Endocrinol. 183 (2004) 145–154.
[47] G.L. Semenza, P.H. Roth, H.M. Fang, G.L. Wang, Transcriptional regulation ofgenes encoding glycolytic enzymes by hypoxia-inducible factor 1, J. Biol.Chem. 269 (1994) 23757–23763.
[48] G.L. Semenza, Regulation of cancer cell metabolism by hypoxia-inducible factor1, Semin. Cancer Biol. 19 (2009) 12–16.
[49] G.L. Semenza, Regulation of metabolism by hypoxia-inducible factor 1, In: ColdSpring Harbor Symposia on Quantitative Biology, 2011.
[50] H.S. Kim, K. Patel, K. Muldoon-Jacobs, K.S. Bisht, N. Aykin-Burns, J.D. Pennington,R. van der Meer, P. Nguyen, J. Savage, K.M. Owens, A. Vassilopoulos, O. Ozden,S.H. Park, K.K. Singh, S.A. Abdulkadir, D.R. Spitz, C.X. Deng, D. Gius, SIRT3 is amitochondria-localized tumor suppressor required for maintenance of mito-chondrial integrity and metabolism during stress, Cancer Cell 17 (2010) 41–52.
[51] E.L. Bell, B.M. Emerling, S.J. Ricoult, L. Guarente, SirT3 suppresses hypoxia induc-ible factor 1alpha and tumor growth by inhibiting mitochondrial ROS produc-tion, Oncogene 30 (2011) 2986–2996.
[52] L.W. Finley, A. Carracedo, J. Lee, A. Souza, A. Egia, J. Zhang, J. Teruya-Feldstein, P.I.Moreira, S.M. Cardoso, C.B. Clish, P.P. Pandolfi, M.C. Haigis, SIRT3 opposes repro-gramming of cancer cell metabolism through HIF1alpha destabilization, CancerCell 19 (2011) 416–428.
[53] R.A. Medina, G.I. Owen, Glucose transporters: expression, regulation and cancer,Biol. Res. 35 (2002) 9–26.
[54] Y. Oka, T. Asano, Y. Shibasaki, J.L. Lin, K. Tsukuda, H. Katagiri, Y. Akanuma, F. Takaku,C-terminal truncated glucose transporter is locked into an inward-facing formwithout transport activity, Nature 345 (1990) 550–553.
[55] D.N. Hebert, A. Carruthers, Glucose transporter oligomeric structure determinestransporter function. Reversible redox-dependent interconversions of tetramer-ic and dimeric GLUT1, J. Biol. Chem. 267 (1992) 23829–23838.
[56] E.K. Cloherty, L.A. Sultzman, R.J. Zottola, A. Carruthers, Net sugar transport is amultistep process. Evidence for cytosolic sugar binding sites in erythrocytes,Biochemistry 34 (1995) 15395–15406.
[57] P.W. Hruz, M.M. Mueckler, Structural analysis of the GLUT1 facilitative glucosetransporter (review), Mol. Membr. Biol. 18 (2001) 183–193.
[58] P.W. Hruz, M.M.Mueckler, Cysteine-scanningmutagenesis of transmembrane seg-ment 7 of the GLUT1 glucose transporter, J. Biol. Chem. 274 (1999) 36176–36180.
[59] M.J. Seatter, S.A. De la Rue, L.M. Porter, G.W. Gould, QLS motif in transmembranehelix VII of the glucose transporter family interacts with the C-1 position ofD-glucose and is involved in substrate selection at the exofacial binding site, Bio-chemistry 37 (1998) 1322–1326.
[60] R.S. Brown, R.L. Wahl, Overexpression of Glut-1 glucose transporter in humanbreast cancer. An immunohistochemical study, Cancer 72 (1993) 2979–2985.
[61] M. Grover-McKay, S.A. Walsh, E.A. Seftor, P.A. Thomas, M.J. Hendrix, Role for glu-cose transporter 1 protein in human breast cancer, Pathol. Oncol. Res. 4 (1998)115–120.
[62] R.S. Haber, A. Rathan, K.R. Weiser, A. Pritsker, S.H. Itzkowitz, C. Bodian, G. Slater,A. Weiss, D.E. Burstein, GLUT1 glucose transporter expression in colorectal car-cinoma: a marker for poor prognosis, Cancer 83 (1998) 34–40.
[63] Y.J. Jun, S.M. Jang, H.L. Han, K.H. Lee, K.S. Jang, S.S. Paik, Clinicopathologic signif-icance of GLUT1 expression and its correlation with Apaf-1 in colorectal adeno-carcinomas, World J. Gastroenterol. 17 (2011) 1866–1873.
[64] T. Amann, C. Hellerbrand, GLUT1 as a therapeutic target in hepatocellular carci-noma, Expert Opin. Ther. Targets 13 (2009) 1411–1427.
[65] T. Amann, U. Maegdefrau, A. Hartmann, A. Agaimy, J. Marienhagen, T.S. Weiss, O.Stoeltzing, C. Warnecke, J. Scholmerich, P.J. Oefner, M. Kreutz, A.K. Bosserhoff, C.Hellerbrand, GLUT1 expression is increased in hepatocellular carcinoma andpromotes tumorigenesis, Am. J. Pathol. 174 (2009) 1544–1552.
[66] K. Kaira, M. Endo, M. Abe, K. Nakagawa, Y. Ohde, T. Okumura, T. Takahashi, H.Murakami, A. Tsuya, Y. Nakamura, T. Naito, I. Hayashi, M. Serizawa, Y. Koh, H.Hanaoka, H. Tominaga, N. Oriuchi, H. Kondo, T. Nakajima, N. Yamamoto, Biologiccorrelation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emissiontomography in thymic epithelial tumors, J. Clin. Oncol. 28 (2010) 3746–3753.
[67] K. Higashi, Y. Ueda, A. Sakurai, X.M. Wang, L. Xu, M. Murakami, H. Seki, M.Oguchi, S. Taki, Y. Nambu, H. Tonami, S. Katsuda, I. Yamamoto, Correlation ofGlut-1 glucose transporter expression with, Eur. J. Nucl. Med. 27 (2000)1778–1785.
[68] J.H. Chung, K.J. Cho, S.S. Lee, H.J. Baek, J.H. Park, G.J. Cheon, C.W. Choi, S.M. Lim,Overexpression of Glut1 in lymphoid follicles correlates with false-positive(18)F-FDG PET results in lung cancer staging, J. Nucl. Med. 45 (2004) 999–1003.
[69] J. Adam, E. Hatipoglu, L. O'Flaherty, N. Ternette, N. Sahgal, H. Lockstone, D.Baban, E. Nye, G.W. Stamp, K. Wolhuter, M. Stevens, R. Fischer, P. Carmeliet,P.H. Maxwell, C.W. Pugh, N. Frizzell, T. Soga, B.M. Kessler, M. El-Bahrawy, P.J.Ratcliffe, P.J. Pollard, Renal cyst formation in Fh1-deficient mice is independentof the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 sig-naling, Cancer Cell 20 (2011) 524–537.
[70] Q.L. Zhou, Z.Y. Jiang, J. Holik, A. Chawla, G.N. Hagan, J. Leszyk, M.P. Czech, Aktsubstrate TBC1D1 regulates GLUT1 expression through the mTOR pathway in3T3-L1 adipocytes, Biochem. J. 411 (2008) 647–655.
[71] C.L. Buller, R.D. Loberg, M.H. Fan, Q. Zhu, J.L. Park, E. Vesely, K. Inoki, K.L. Guan,F.C. Brosius III, A GSK-3/TSC2/mTOR pathway regulates glucose uptake andGLUT1 glucose transporter expression, Am. J. Physiol. Cell Physiol. 295 (2008)C836–C843.
[72] H. Tong, K. Imahashi, C. Steenbergen, E. Murphy, Phosphorylation of glycogensynthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase‐dependent pathway is cardioprotective, Circ. Res. 90 (2002) 377–379.
[73] K. Inoki, H. Ouyang, T. Zhu, C. Lindvall, Y. Wang, X. Zhang, Q. Yang, C. Bennett,Y. Harada, K. Stankunas, C.Y. Wang, X. He, O.A. MacDougald, M. You, B.O.Williams, K.L. Guan, TSC2 integrates Wnt and energy signals via a coordinatedphosphorylation by AMPK and GSK3 to regulate cell growth, Cell 126 (2006)955–968.
[74] N. Hay, N. Sonenberg, Upstream and downstream of mTOR, Genes Dev. 18(2004) 1926–1945.
[75] C.S. Beevers, F. Li, L. Liu, S. Huang, Curcumin inhibits the mammalian target ofrapamycin-mediated signaling pathways in cancer cells, Int. J. Cancer 119(2006) 757–764.
[76] C. Tokunaga, K. Yoshino, K. Yonezawa, mTOR integrates amino acid- andenergy-sensing pathways, Biochem. Biophys. Res. Commun. 313 (2004) 443–446.
[77] J.B. Brugarolas, F. Vazquez, A. Reddy, W.R. Sellers, W.G. Kaelin Jr., TSC2 regulatesVEGF through mTOR-dependent and -independent pathways, Cancer Cell 4(2003) 147–158.
[78] C.L. Buller, C.W. Heilig, F.C. Brosius III, GLUT1 enhances mTOR activity indepen-dently of TSC2 and AMPK, Am. J. Physiol. Renal Physiol. 301 (2011) F588–F596.
[79] A. Barthel, S.T. Okino, J. Liao, K. Nakatani, J. Li, J.P. Whitlock Jr., R.A. Roth, Regula-tion of GLUT1 gene transcription by the serine/threonine kinase Akt1, J. Biol.Chem. 274 (1999) 20281–20286.
[80] C. Taha, Z. Liu, J. Jin, H. Al-Hasani, N. Sonenberg, A. Klip, Opposite translationalcontrol of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin.

382 J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol3-kinase in GLUT1 mRNA translation, J. Biol. Chem. 274 (1999) 33085–33091.
[81] K. Kawauchi, K. Araki, K. Tobiume, N. Tanaka, p53 regulates glucose metabolismthrough an IKK–NF-kappaB pathway and inhibits cell transformation, Nat. CellBiol. 10 (2008) 611–618.
[82] B.F. Ren, L.F. Deng, J. Wang, Y.P. Zhu, L. Wei, Q. Zhou, Hypoxia regulation of facil-itated glucose transporter-1 and glucose transporter-3 in mouse chondrocytesmediated by HIF-1alpha, Joint Bone Spine 75 (2008) 176–181.
[83] A. Mobasheri, S. Richardson, R. Mobasheri, M. Shakibaei, J.A. Hoyland, Hypoxiainducible factor-1 and facilitative glucose transporters GLUT1 and GLUT3: puta-tive molecular components of the oxygen and glucose sensing apparatus in ar-ticular chondrocytes, Histol. Histopathol. 20 (2005) 1327–1338.
[84] M.U. Baumann, S. Zamudio, N.P. Illsley, Hypoxic upregulation of glucose trans-porters in BeWo choriocarcinoma cells is mediated by hypoxia-induciblefactor-1, Am. J. Physiol. Cell Physiol. 293 (2007) C477–C485.
[85] A. Evans, V. Bates, H. Troy, S. Hewitt, S. Holbeck, Y.L. Chung, R. Phillips, M. Stubbs,J. Griffiths, R. Airley, Glut-1 as a therapeutic target: increased chemoresistanceand HIF-1-independent link with cell turnover is revealed through COMPAREanalysis and metabolomic studies, Cancer Chemother. Pharmacol. 61 (2008)377–393.
[86] X.M. Luo, S.H. Zhou, J. Fan, Glucose transporter-1 as a new therapeutic target inlaryngeal carcinoma, J. Int. Med. Res. 38 (2010) 1885–1892.
[87] Y. Liu, Y. Cao, W. Zhang, S. Bergmeier, Y. Qian, H. Akbar, R. Colvin, J. Ding, L. Tong,S. Wu, J. Hines, X. Chen, A small molecule inhibitor of glucose transporter 1down-regulates glycolysis, induces cell cycle arrest, and inhibits cancer cellgrowth in vitro and in vivo, Mol. Cancer Ther. 11 (2012) 1672–1682.
[88] A. Minchenko, I. Leshchinsky, I. Opentanova, N. Sang, V. Srinivas, V. Armstead, J. Caro,Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in theWarburg effect,J. Biol. Chem. 277 (2002) 6183–6187.
[89] M. Emmerling, J.E. Bailey, U. Sauer, Glucose catabolism of Escherichia coli strainswith increased activity and altered regulation of key glycolytic enzymes, Metab.Eng. 1 (1999) 117–127.
[90] J. Hauf, F.K. Zimmermann, S. Muller, Simultaneous genomic overexpression ofseven glycolytic enzymes in the yeast Saccharomyces cerevisiae, Enzyme Microb.Technol. 26 (2000) 688–698.
[91] A. Smerc, E. Sodja, M. Legisa, Posttranslational modification of 6-phosphofructo-1-kinase as an important feature of cancer metabolism, PLoS One 6 (2011) e19645.
[92] S.P. Mathupala, Y.H. Ko, P.L. Pedersen, Hexokinase-2 bound to mitochondria:cancer's stygian link to the “Warburg Effect” and a pivotal target for effectivetherapy, Semin. Cancer Biol. 19 (2009) 17–24.
[93] N. Majewski, V. Nogueira, P. Bhaskar, P.E. Coy, J.E. Skeen, K. Gottlob, N.S.Chandel, C.B. Thompson, R.B. Robey, N. Hay, Hexokinase-mitochondria interac-tion mediated by Akt is required to inhibit apoptosis in the presence or absenceof Bax and Bak, Mol. Cell 16 (2004) 819–830.
[94] N. Majewski, V. Nogueira, R.B. Robey, N. Hay, Akt inhibits apoptosis downstreamof BID cleavage via a glucose-dependent mechanism involving mitochondrialhexokinases, Mol. Cell. Biol. 24 (2004) 730–740.
[95] J.W. Kim, C.V. Dang, Multifaceted roles of glycolytic enzymes, Trends Biochem.Sci. 30 (2005) 142–150.
[96] P.L. Pedersen, Voltage dependent anion channels (VDACs): a brief introductionwith a focus on the outer mitochondrial compartment's roles together withhexokinase-2 in the “Warburg effect” in cancer, J. Bioenerg. Biomembr. 40(2008) 123–126.
[97] S.P. Mathupala, C. Heese, P.L. Pedersen, Glucose catabolism in cancer cells. Thetype II hexokinase promoter contains functionally active response elementsfor the tumor suppressor p53, J. Biol. Chem. 272 (1997) 22776–22780.
[98] P.L. Pedersen, S. Mathupala, A. Rempel, J.F. Geschwind, Y.H. Ko, Mitochondrialbound type II hexokinase: a key player in the growth and survival of many can-cers and an ideal prospect for therapeutic intervention, Biochim. Biophys. Acta1555 (2002) 14–20.
[99] G.A. Dunaway, A review of animal phosphofructokinase isozymes with an em-phasis on their physiological role, Mol. Cell. Biochem. 52 (1983) 75–91.
[100] G. Wegener, U. Krause, Different modes of activating phosphofructokinase, a keyregulatory enzyme of glycolysis, in working vertebrate muscle, Biochem. Soc.Trans. 30 (2002) 264–270.
[101] R.A. Poorman, A. Randolph, R.G. Kemp, R.L. Heinrikson, Evolution of phospho-fructokinase—gene duplication and creation of new effector sites, Nature 309(1984) 467–469.
[102] T. Kawaguchi, R.L. Veech, K. Uyeda, Regulation of energymetabolism inmacrophagesduring hypoxia. Roles of fructose 2,6-bisphosphate and ribose 1,5-bisphosphate, J.Biol. Chem. 276 (2001) 28554–28561.
[103] D.A. Okar, A.J. Lange, Fructose-2,6-bisphosphate and control of carbohydratemetabolism in eukaryotes, Biofactors 10 (1999) 1–14.
[104] D.A. Okar, A. Manzano, A. Navarro-Sabate, L. Riera, R. Bartrons, A.J. Lange,PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate, Trends Biochem. Sci. 26 (2001) 30–35.
[105] S.J. Pilkis, T.H. Claus, I.J. Kurland, A.J. Lange, 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a metabolic signaling enzyme, Annu. Rev. Biochem. 64 (1995)799–835.
[106] J. Chesney, R. Mitchell, F. Benigni, M. Bacher, L. Spiegel, Y. Al-Abed, J.H. Han, C.Metz, R. Bucala, An inducible gene product for 6-phosphofructo-2-kinase withan AU-rich instability element: role in tumor cell glycolysis and the Warburg ef-fect, Proc. Natl. Acad. Sci. U. S. A. 96 (1999) 3047–3052.
[107] A. Manzano, J.L. Rosa, F. Ventura, J.X. Perez, M. Nadal, X. Estivill, S. Ambrosio, J.Gil, R. Bartrons, Molecular cloning, expression, and chromosomal localization
of a ubiquitously expressed human 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene (PFKFB3), Cytogenet. Cell Genet. 83 (1998) 214–217.
[108] J. Algaier, K. Uyeda, Molecular cloning, sequence analysis, and expression of ahuman liver cDNA coding for fructose-6-P,2-kinase:fructose-2,6-bisphosphatase,Biochem. Biophys. Res. Commun. 153 (1988) 328–333.
[109] D. Heine-Suner,M.A. Diaz-Guillen, A.J. Lange, S. Rodriguez de Cordoba, Sequence andstructure of the human 6-phosphofructo-2-kinase/fructose-2,6-bisphosphataseheart isoform gene (PFKFB2), Eur. J. Biochem. 254 (1998) 103–110.
[110] A. Sakai, M. Kato, M. Fukasawa, M. Ishiguro, E. Furuya, R. Sakakibara, Cloning ofcDNA encoding for a novel isozyme of fructose 6-phosphate, 2-kinase/fructose2,6-bisphosphatase from human placenta, J. Biochem. 119 (1996) 506–511.
[111] R. Sakakibara, M. Kato, N. Okamura, T. Nakagawa, Y. Komada, N. Tominaga, M.Shimojo, M. Fukasawa, Characterization of a human placental fructose-6-phosphate,2-kinase/fructose-2,6-bisphosphatase, J. Biochem. 122 (1997) 122–128.
[112] J. Boada, T. Roig, X. Perez, A. Gamez, R. Bartrons, M. Cascante, J. Bermudez, Cellsoverexpressing fructose-2,6-bisphosphatase showed enhanced pentose phosphatepathway flux and resistance to oxidative stress, FEBS Lett. 480 (2000) 261–264.
[113] T. Hirata, M. Watanabe, S. Miura, K. Ijichi, M. Fukasawa, R. Sakakibara, Inhibitionof tumor cell growth by a specific 6-phosphofructo-2-kinase inhibitor,N-bromoacetylethanolamine phosphate, and its analogues, Biosci. Biotechnol.Biochem. 64 (2000) 2047–2052.
[114] T. Atsumi, J. Chesney, C. Metz, L. Leng, S. Donnelly, Z. Makita, R. Mitchell, R. Bucala,High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase(iPFK-2; PFKFB3) in human cancers, Cancer Res. 62 (2002) 5881–5887.
[115] J.X. Perez, T. Roig, A. Manzano, M. Dalmau, J. Boada, F. Ventura, J.L. Rosa, J.Bermudez, R. Bartrons, Overexpression of fructose 2,6-bisphosphatase decreasesglycolysis and delays cell cycle progression, Am. J. Physiol. Cell Physiol. 279(2000) C1359–C1365.
[116] R.F. Kletzien, P.K. Harris, L.A. Foellmi, Glucose-6-phosphate dehydrogenase: a“housekeeping” enzyme subject to tissue-specific regulation by hormones, nu-trients, and oxidant stress, FASEB J. 8 (1994) 174–181.
[117] A. Fico, F. Paglialunga, L. Cigliano, P. Abrescia, P. Verde, G. Martini, I. Iaccarino, S.Filosa, Glucose-6-phosphate dehydrogenase plays a crucial role in protectionfrom redox-stress-induced apoptosis, Cell Death Differ. 11 (2004) 823–831.
[118] P. Ruiz-Lozano, M.L. Hixon, M.W.Wagner, A.I. Flores, S. Ikawa, A.S. Baldwin Jr., K.R.Chien, A. Gualberto, p53 is a transcriptional activator of the muscle-specific phos-phoglycerate mutase gene and contributes in vivo to the control of its cardiac ex-pression, Cell Growth Differ. 10 (1999) 295–306.
[119] C.V. Dang, G.L. Semenza, Oncogenic alterations of metabolism, Trends Biochem.Sci. 24 (1999) 68–72.
[120] T. Yamasaki, H. Nakajima, N. Kono, K. Hotta, K. Yamada, E. Imai, M. Kuwajima,T. Noguchi, T. Tanaka, S. Tarui, Structure of the entire human musclephosphofructokinase-encoding gene: a two-promoter system, Gene 104(1991) 277–282.
[121] A. Elson, D. Levanon, M. Brandeis, N. Dafni, Y. Bernstein, E. Danciger, Y. Groner,The structure of the human liver-type phosphofructokinase gene, Genomics 7(1990) 47–56.
[122] K. Eto, H. Sakura, K. Yasuda, T. Hayakawa, E. Kawasaki, R. Moriuchi, S. Nagataki,Y. Yazaki, T. Kadowaki, Cloning of a complete protein-coding sequence of humanplatelet-type phosphofructokinase isozyme from pancreatic islet, Biochem.Biophys. Res. Commun. 198 (1994) 990–998.
[123] S. Vora, R. Oskam, G.E. Staal, Isoenzymes of phosphofructokinase in the rat.Demonstration of the three non-identical subunits by biochemical, immuno-chemical and kinetic studies, Biochem. J. 229 (1985) 333–341.
[124] G.A. Dunaway, T.P. Kasten, T. Sebo, R. Trapp, Analysis of the phosphofructokinasesubunits and isoenzymes in human tissues, Biochem. J. 251 (1988) 677–683.
[125] G.E. Staal, A. Kalff, E.C. Heesbeen, C.W. van Veelen, G. Rijksen, Subunit composi-tion, regulatory properties, and phosphorylation of phosphofructokinase fromhuman gliomas, Cancer Res. 47 (1987) 5047–5051.
[126] A. Marin-Hernandez, S. Rodriguez-Enriquez, P.A. Vital-Gonzalez, F.L.Flores-Rodriguez, M. Macias-Silva, M. Sosa-Garrocho, R. Moreno-Sanchez, De-termining and understanding the control of glycolysis in fast-growth tumorcells. Flux control by an over-expressed but strongly product-inhibited hexoki-nase, FEBS J. 273 (2006) 1975–1988.
[127] M. Legisa, M. Mattey, Changes in primary metabolism leading to citric acid over-flow in Aspergillus niger, Biotechnol. Lett. 29 (2007) 181–190.
[128] S.Mesojednik,M. Legisa, Posttranslationalmodification of 6-phosphofructo-1-kinasein Aspergillus niger, Appl. Environ. Microbiol. 71 (2005) 1425–1432.
[129] T. Mlakar, M. Legisa, Citrate inhibition-resistant form of 6-phosphofructo-1-kinasefrom Aspergillus niger, Appl. Environ. Microbiol. 72 (2006) 4515–4521.
[130] M. Capuder, T. Solar, M. Bencina, M. Legisa, Highly active, citrate inhibition resis-tant form of Aspergillus niger 6-phosphofructo-1-kinase encoded by a modifiedpfkA gene, J. Biotechnol. 144 (2009) 51–57.
[131] F.J. Kayne, N.C. Price, Amino acid effector binding to rabbit muscle pyruvate ki-nase, Arch. Biochem. Biophys. 159 (1973) 292–296.
[132] H. Tsutsumi, K. Tani, H. Fujii, S. Miwa, Expression of L- and M-type pyruvate ki-nase in human tissues, Genomics 2 (1988) 86–89.
[133] H.R. Christofk, M.G. Vander Heiden, M.H. Harris, A. Ramanathan, R.E. Gerszten, R.Wei, M.D. Fleming, S.L. Schreiber, L.C. Cantley, The M2 splice isoform of pyruvatekinase is important for cancer metabolism and tumour growth, Nature 452(2008) 230–233.
[134] H.R. Christofk, M.G. Vander Heiden, N. Wu, J.M. Asara, L.C. Cantley, Pyruvate ki-nase M2 is a phosphotyrosine-binding protein, Nature 452 (2008) 181–186.
[135] B. Kefas, L. Comeau, N. Erdle, E. Montgomery, S. Amos, B. Purow, Pyruvate kinaseM2 is a target of the tumor-suppressive microRNA-326 and regulates the surviv-al of glioma cells, Neuro Oncol. 12 (2010) 1102–1112.

383J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
[136] B.C. Yoo, J.L. Ku, S.H. Hong, Y.K. Shin, S.Y. Park, H.K. Kim, J.G. Park, Decreased py-ruvate kinase M2 activity linked to cisplatin resistance in human gastric carcino-ma cell lines, Int. J. Cancer 108 (2004) 532–539.
[137] E. Martinez-Balibrea, C. Plasencia, A. Gines, A. Martinez-Cardus, E. Musulen, R.Aguilera, J.L. Manzano, N. Neamati, A. Abad, A proteomic approach links de-creased pyruvate kinase M2 expression to oxaliplatin resistance in patientswith colorectal cancer and in human cell lines, Mol. Cancer Ther. 8 (2009)771–778.
[138] K. Akhtar, V. Gupta, A. Koul, N. Alam, R. Bhat, R.N. Bamezai, Differential behaviorof missense mutations in the intersubunit contact domain of the human pyru-vate kinase M2 isozyme, J. Biol. Chem. 284 (2009) 11971–11981.
[139] V. Gupta, P. Kalaiarasan, M. Faheem, N. Singh, M.A. Iqbal, R.N. Bamezai, Domi-nant negative mutations affect oligomerization of human pyruvate kinase M2isozyme and promote cellular growth and polyploidy, J. Biol. Chem. 285(2010) 16864–16873.
[140] L.G. Korotchkina, M.S. Patel, Site specificity of four pyruvate dehydrogenase ki-nase isoenzymes toward the three phosphorylation sites of human pyruvate de-hydrogenase, J. Biol. Chem. 276 (2001) 37223–37229.
[141] L.G. Korotchkina, M.S. Patel, Probing the mechanism of inactivation of humanpyruvate dehydrogenase by phosphorylation of three sites, J. Biol. Chem. 276(2001) 5731–5738.
[142] M. Kato, R.M. Wynn, J.L. Chuang, S.C. Tso, M. Machius, J. Li, D.T. Chuang, Structur-al basis for inactivation of the human pyruvate dehydrogenase complex byphosphorylation: role of disordered phosphorylation loops, Structure 16(2008) 1849–1859.
[143] M.C. Sugden, M.J. Holness, Recent advances in mechanisms regulating glucose ox-idation at the level of the pyruvate dehydrogenase complex by PDKs, Am. J. Physiol.Endocrinol. Metab. 284 (2003) E855–E862.
[144] M. Board, S. Humm, E.A. Newsholme, Maximum activities of key enzymes ofglycolysis, glutaminolysis, pentose phosphate pathway and tricarboxylic acidcycle in normal, neoplastic and suppressed cells, Biochem. J. 265 (1990)503–509.
[145] K.M. Popov, N.Y. Kedishvili, Y. Zhao, R. Gudi, R.A. Harris, Molecular cloning of thep45 subunit of pyruvate dehydrogenase kinase, J. Biol. Chem. 269 (1994)29720–29724.
[146] J.W. Kim, I. Tchernyshyov, G.L. Semenza, C.V. Dang, HIF-1-mediated expressionof pyruvate dehydrogenase kinase: a metabolic switch required for cellular ad-aptation to hypoxia, Cell Metab. 3 (2006) 177–185.
[147] M.I. Koukourakis, A. Giatromanolaki, E. Sivridis, K.C. Gatter, A.L. Harris, Pyruvatedehydrogenase and pyruvate dehydrogenase kinase expression in non small celllung cancer and tumor-associated stroma, Neoplasia 7 (2005) 1–6.
[148] S. Bonnet, S.L. Archer, J. Allalunis-Turner, A. Haromy, C. Beaulieu, R. Thompson,C.T. Lee, G.D. Lopaschuk, L. Puttagunta, G. Harry, K. Hashimoto, C.J. Porter, M.A.Andrade, B. Thebaud, E.D. Michelakis, A mitochondria-K+ channel axis issuppressed in cancer and its normalization promotes apoptosis and inhibits can-cer growth, Cancer Cell 11 (2007) 37–51.
[149] M. Kato, J. Li, J.L. Chuang, D.T. Chuang, Distinct structural mechanisms for inhibi-tion of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate,and radicicol, Structure 15 (2007) 992–1004.
[150] C.W. Lu, S.C. Lin, C.W. Chien, C.T. Lee, B.W. Lin, J.C. Lee, S.J. Tsai, Overexpressionof pyruvate dehydrogenase kinase 3 increases drug resistance and early recur-rence in colon cancer, Am. J. Pathol. 179 (2011) 1405–1414.
[151] H. Lauble, M.C. Kennedy, H. Beinert, C.D. Stout, Crystal structures of aconitasewith isocitrate and nitroisocitrate bound, Biochemistry 31 (1992) 2735–2748.
[152] H. Lauble, M.C. Kennedy, H. Beinert, C.D. Stout, Crystal structures of aconitasewith trans-aconitate and nitrocitrate bound, J. Mol. Biol. 237 (1994) 437–451.
[153] M.E. Mycielska, T.P. Broke-Smith, C.P. Palmer, R. Beckerman, T. Nastos, K.Erguler, M.B. Djamgoz, Citrate enhances in vitro metastatic behaviours ofPC-3M human prostate cancer cells: status of endogenous citrate and depen-dence on aconitase and fatty acid synthase, Int. J. Biochem. Cell Biol. 38 (2006)1766–1777.
[154] K.K. Singh, M.M. Desouki, R.B. Franklin, L.C. Costello, Mitochondrial aconitaseand citrate metabolism in malignant and nonmalignant human prostate tissues,Mol. Cancer 5 (2006) 14.
[155] K.H. Tsui, T.H. Feng, Y.F. Lin, P.L. Chang, H.H. Juang, p53 downregulates the geneexpression of mitochondrial aconitase in human prostate carcinoma cells, Pros-tate 71 (2011) 62–70.
[156] D.W. Parsons, S. Jones, X. Zhang, J.C. Lin, R.J. Leary, P. Angenendt, P. Mankoo, H.Carter, I.M. Siu, G.L. Gallia, A. Olivi, R. McLendon, B.A. Rasheed, S. Keir, T.Nikolskaya, Y. Nikolsky, D.A. Busam, H. Tekleab, L.A. Diaz Jr., J. Hartigan, D.R.Smith, R.L. Strausberg, S.K. Marie, S.M. Shinjo, H. Yan, G.J. Riggins, D.D. Bigner,R. Karchin, N. Papadopoulos, G. Parmigiani, B. Vogelstein, V.E. Velculescu, K.W.Kinzler, An integrated genomic analysis of human glioblastoma multiforme, Sci-ence 321 (2008) 1807–1812.
[157] H. Yan, D.W. Parsons, G. Jin, R. McLendon, B.A. Rasheed, W. Yuan, I. Kos, I.Batinic-Haberle, S. Jones, G.J. Riggins, H. Friedman, A. Friedman, D. Reardon, J.Herndon, K.W. Kinzler, V.E. Velculescu, B. Vogelstein, D.D. Bigner, IDH1 andIDH2 mutations in gliomas, N. Engl. J. Med. 360 (2009) 765–773.
[158] P.S.Ward, J. Patel, D.R.Wise, O. Abdel-Wahab, B.D. Bennett, H.A. Coller, J.R. Cross, V.R.Fantin, C.V. Hedvat, A.E. Perl, J.D. Rabinowitz, M. Carroll, S.M. Su, K.A. Sharp, R.L.Levine, C.B. Thompson, The common feature of leukemia-associated IDH1 andIDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarateto 2-hydroxyglutarate, Cancer Cell 17 (2010) 225–234.
[159] M. Kranendijk, E.A. Struys, E. van Schaftingen, K.M. Gibson, W.A. Kanhai, M.S.van der Knaap, J. Amiel, N.R. Buist, A.M. Das, J.B. de Klerk, A.S. Feigenbaum,D.K. Grange, F.C. Hofstede, E. Holme, E.P. Kirk, S.H. Korman, E. Morava, A.
Morris, J. Smeitink, R.N. Sukhai, H. Vallance, C. Jakobs, G.S. Salomons, IDH2 mu-tations in patients with D-2-hydroxyglutaric aciduria, Science 330 (2010) 336.
[160] L. Dang, D.W.White, S. Gross, B.D. Bennett,M.A. Bittinger, E.M.Driggers, V.R. Fantin,H.G. Jang, S. Jin, M.C. Keenan, K.M. Marks, R.M. Prins, P.S. Ward, K.E. Yen, L.M. Liau,J.D. Rabinowitz, L.C. Cantley, C.B. Thompson, M.G. Vander Heiden, S.M. Su,Cancer-associated IDH1 mutations produce 2-hydroxyglutarate, Nature 465(2010) 966.
[161] K.S. Oyedotun, B.D. Lemire, The quaternary structure of the Saccharomycescerevisiae succinate dehydrogenase. Homology modeling, cofactor docking, andmolecular dynamics simulation studies, J. Biol. Chem. 279 (2004) 9424–9431.
[162] B.E. Baysal, On the association of succinate dehydrogenase mutations with he-reditary paraganglioma, Trends Endocrinol. Metab. 14 (2003) 453–459.
[163] H.P. Neumann, C. Pawlu, M. Peczkowska, B. Bausch, S.R. McWhinney, M.Muresan, M. Buchta, G. Franke, J. Klisch, T.A. Bley, S. Hoegerle, C.C. Boedeker,G. Opocher, J. Schipper, A. Januszewicz, C. Eng, Distinct clinical features of para-ganglioma syndromes associated with SDHB and SDHD gene mutations, JAMA292 (2004) 943–951.
[164] P.J. Pollard, N.C. Wortham, I.P. Tomlinson, The TCA cycle and tumorigenesis: theexamples of fumarate hydratase and succinate dehydrogenase, Ann. Med. 35(2003) 632–639.
[165] C. Eng, M. Kiuru, M.J. Fernandez, L.A. Aaltonen, A role for mitochondrial enzymesin inherited neoplasia and beyond, Nat. Rev. Cancer 3 (2003) 193–202.
[166] P. Rustin, A. Rotig, Inborn errors of complex II—unusual human mitochondrialdiseases, Biochim. Biophys. Acta 1553 (2002) 117–122.
[167] W. Habano, T. Sugai, S. Nakamura, N. Uesugi, T. Higuchi, M. Terashima, S.Horiuchi, Reduced expression and loss of heterozygosity of the SDHD gene incolorectal and gastric cancer, Oncol. Rep. 10 (2003) 1375–1380.
[168] A.P. Gimenez-Roqueplo, J. Favier, P. Rustin, J.J. Mourad, P.F. Plouin, P. Corvol, A.Rotig, X. Jeunemaitre, The R22X mutation of the SDHD gene in hereditary para-ganglioma abolishes the enzymatic activity of complex II in the mitochondrialrespiratory chain and activates the hypoxia pathway, Am. J. Hum. Genet. 69(2001) 1186–1197.
[169] A.P. Gimenez-Roqueplo, J. Favier, P. Rustin, C. Rieubland, M. Crespin, V. Nau, P.Khau Van Kien, P. Corvol, P.F. Plouin, X. Jeunemaitre, Mutations in the SDHBgene are associated with extra-adrenal and/or malignant phaeochromocytomas,Cancer Res. 63 (2003) 5615–5621.
[170] A.P. Gimenez-Roqueplo, J. Favier, P. Rustin, C. Rieubland, V. Kerlan, P.F. Plouin, A.Rotig, X. Jeunemaitre, Functional consequences of a SDHB gene mutation in anapparently sporadic pheochromocytoma, J. Clin. Endocrinol. Metab. 87 (2002)4771–4774.
[171] M.A. Selak, S.M. Armour, E.D. MacKenzie, H. Boulahbel, D.G. Watson, K.D.Mansfield, Y. Pan, M.C. Simon, C.B. Thompson, E. Gottlieb, Succinate links TCAcycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase,Cancer Cell 7 (2005) 77–85.
[172] I.P. Tomlinson, N.A. Alam, A.J. Rowan, E. Barclay, E.E. Jaeger, D. Kelsell, I. Leigh, P.Gorman, H. Lamlum, S. Rahman, R.R. Roylance, S. Olpin, S. Bevan, K. Barker, N.Hearle, R.S. Houlston, M. Kiuru, R. Lehtonen, A. Karhu, S. Vilkki, P. Laiho, C.Eklund, O. Vierimaa, K. Aittomaki, M. Hietala, P. Sistonen, A. Paetau, R.Salovaara, R. Herva, V. Launonen, L.A. Aaltonen, Germline mutations in FH pre-dispose to dominantly inherited uterine fibroids, skin leiomyomata and papil-lary renal cell cancer, Nat. Genet. 30 (2002) 406–410.
[173] A. Martinez-Mir, B. Glaser, G.S. Chuang, L. Horev, A. Waldman, D.E. Engler, D.Gordon, L.J. Spelman, I. Hatzibougias, J. Green, A.M. Christiano, A. Zlotogorski,Germline fumarate hydratase mutations in families with multiple cutaneousand uterine leiomyomata, J. Investig. Dermatol. 121 (2003) 741–744.
[174] S. Sudarshan, K. Shanmugasundaram, S.L. Naylor, S. Lin, C.B. Livi, C.F. O'Neill, D.J.Parekh, I.T. Yeh, L.Z. Sun, K. Block, Reduced expression of fumarate hydratase inclear cell renal cancer mediates HIF-2alpha accumulation and promotes migra-tion and invasion, PLoS One 6 (2011) e21037.
[175] C. Bardella, M. El-Bahrawy,N. Frizzell, J. Adam, N. Ternette, E. Hatipoglu, K. Howarth,L. O'Flaherty, I. Roberts, G. Turner, J. Taylor, K. Giaslakiotis, V.M. Macaulay, A.L.Harris, A. Chandra, H.J. Lehtonen, V. Launonen, L.A. Aaltonen, C.W. Pugh, R. Mihai,D. Trudgian, B. Kessler, J.W. Baynes, P.J. Ratcliffe, I.P. Tomlinson, P.J. Pollard, Aberrantsuccination of proteins in fumarate hydratase-deficient mice and HLRCC patients isa robust biomarker of mutation status, J. Pathol. 225 (2011) 4–11.
[176] H. Ashrafian, G. Czibik, M. Bellahcene, D. Aksentijevic, A.C. Smith, S.J. Mitchell,M.S. Dodd, J. Kirwan, J.J. Byrne, C. Ludwig, H. Isackson, A. Yavari, N.B. Stottrup,H. Contractor, T.J. Cahill, N. Sahgal, D.R. Ball, R.I. Birkler, I. Hargreaves, D.A.Tennant, J. Land, C.A. Lygate, M. Johannsen, R.K. Kharbanda, S. Neubauer, C.Redwood, R. de Cabo, I. Ahmet, M. Talan, U.L. Gunther, A.J. Robinson, M.R.Viant, P.J. Pollard, D.J. Tyler, H. Watkins, Fumarate is cardioprotective via activa-tion of the Nrf2 antioxidant pathway, Cell Metab. 15 (2012) 361–371.
[177] R.J. Smith, Glutamine metabolism and its physiologic importance, J. Parenter.Enteral Nutr. 14 (1990) 40S–44S.
[178] P. Newsholme, J. Procopio, M.M. Lima, T.C. Pithon-Curi, R. Curi, Glutamine andglutamate—their central role in cell metabolism and function, Cell Biochem.Funct. 21 (2003) 1–9.
[179] R. Curi, C.J. Lagranha, S.Q. Doi, D.F. Sellitti, J. Procopio, T.C. Pithon-Curi, M. Corless, P.Newsholme,Molecularmechanisms of glutamine action, J. Cell. Physiol. 204 (2005)392–401.
[180] A. Cassago, A.P. Ferreira, I.M. Ferreira, C. Fornezari, E.R. Gomes, K.S. Greene, H.M.Pereira, R.C. Garratt, S.M. Dias, A.L. Ambrosio, Mitochondrial localization andstructure-based phosphate activation mechanism of Glutaminase C with implica-tions for cancer metabolism, Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 1092–1097.
[181] G. Wu, Y.Z. Fang, S. Yang, J.R. Lupton, N.D. Turner, Glutathione metabolism andits implications for health, J. Nutr. 134 (2004) 489–492.

384 J.-Q. Chen, J. Russo / Biochimica et Biophysica Acta 1826 (2012) 370–384
[182] C. Des Rosiers, L. Di Donato, B. Comte, A. Laplante, C. Marcoux, F. David, C.A.Fernandez, H. Brunengraber, Isotopomer analysis of citric acid cycle and gluco-neogenesis in rat liver. Reversibility of isocitrate dehydrogenase and involve-ment of ATP-citrate lyase in gluconeogenesis, J. Biol. Chem. 270 (1995)10027–10036.
[183] H. Yoo, M.R. Antoniewicz, G. Stephanopoulos, J.K. Kelleher, Quantifying reductivecarboxylation flux of glutamine to lipid in a brown adipocyte cell line, J. Biol.Chem. 283 (2008) 20621–20627.
[184] R.J. DeBerardinis, A. Mancuso, E. Daikhin, I. Nissim, M. Yudkoff, S. Wehrli, C.B.Thompson, Beyond aerobic glycolysis: transformed cells can engage in gluta-mine metabolism that exceeds the requirement for protein and nucleotide syn-thesis, Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 19345–19350.
[185] C.M. Metallo, P.A. Gameiro, E.L. Bell, K.R. Mattaini, J. Yang, K. Hiller, C.M. Jewell,Z.R. Johnson, D.J. Irvine, L. Guarente, J.K. Kelleher, M.G. Vander Heiden, O.Iliopoulos, G. Stephanopoulos, Reductive glutamine metabolism by IDH1 medi-ates lipogenesis under hypoxia, Nature 481 (2012) 380–384.
[186] N.S. Forbes, A.L. Meadows, D.S. Clark, H.W. Blanch, Estradiol stimulates the bio-synthetic pathways of breast cancer cells: detection by metabolic flux analysis,Metab. Eng. 8 (2006) 639–652.
[187] J.W. Erickson, R.A. Cerione, Glutaminase: a hot spot for regulation of cancer cellmetabolism? Oncotarget 1 (2010) 734–740.
[188] S.L. Colombo, M. Palacios-Callender, N. Frakich, S. Carcamo, I. Kovacs, S.Tudzarova, S. Moncada, Molecular basis for the differential use of glucose andglutamine in cell proliferation as revealed by synchronized HeLa cells, Proc.Natl. Acad. Sci. U. S. A. 108 (2011) 21069–21074.
[189] C. Perez-Gomez, J.M. Mates, P.M. Gomez-Fabre, A. del Castillo-Olivares, F.J.Alonso, J. Marquez, Genomic organization and transcriptional analysis of thehuman l-glutaminase gene, Biochem. J. 370 (2003) 771–784.
[190] J.A. Campos-Sandoval, A.R. Lopez de laOliva, C. Lobo, J.A. Segura, J.M.Mates, F.J. Alonso,J. Marquez, Expression of functional human glutaminase in baculovirus system: affin-ity purification, kinetic and molecular characterization, Int. J. Biochem. Cell Biol. 39(2007) 765–773.
[191] D.R. Wise, C.B. Thompson, Glutamine addiction: a new therapeutic target in can-cer, Trends Biochem. Sci. 35 (2010) 427–433.
[192] K.N. Rajagopalan, R.J. DeBerardinis, Role of glutamine in cancer: therapeutic andimaging implications, J. Nucl. Med. 52 (2011) 1005–1008.
[193] J.B. Wang, J.W. Erickson, R. Fuji, S. Ramachandran, P. Gao, R. Dinavahi, K.F.Wilson, A.L. Ambrosio, S.M. Dias, C.V. Dang, R.A. Cerione, Targeting mitochondri-al glutaminase activity inhibits oncogenic transformation, Cancer Cell 18 (2010)207–219.
[194] S. Roy, S. Ghosh, P. Mallick, P. Maity, Acivicin with glutaminase regulates prolif-eration and invasion of human MCF-7 and OAW-42 cells—an in vitro study, In-dian J. Exp. Biol. 46 (2008) 22–26.
[195] M. Hollstein, D. Sidransky, B. Vogelstein, C.C. Harris, p53 mutations in humancancers, Science 253 (1991) 49–53.
[196] P.S. Ward, C.B. Thompson, Metabolic reprogramming: a cancer hallmark evenWarburg did not anticipate, Cancer Cell 21 (2012) 297–308.
[197] K. Kaira, M. Endo, T. Shukuya, H. Kenmotsu, T. Naito, A. Ono, A. Tsuya, Y.Nakamura, T. Takahashi, H. Murakami, H. Kondo, T. Nakajima, N. Yamamoto,18F-FDG uptake on PET could be a predictive marker of Excision RepairCross-Complementation Group 1 (ERCC1) expression in patients with thoracicneoplasms? Neoplasma (2012) 1–2.
[198] K. Kaira, M. Serizawa, Y. Koh, T. Takahashi, H. Hanaoka, N. Oriuchi, M. Endo, H.Kondo, T. Nakajima, N. Yamamoto, Relationship between (18)F-FDG uptake onpositron emission tomography and molecular biology in malignant pleural me-sothelioma, Eur. J. Cancer 48 (2012) 1244–1254.



![TC ATUC 04 08.ppt [Kompatibilitätsmodus] · Internal Recirculation (IRC) Available on TCA/TCRAvailable on TCA/TCR Ιντερναλ φλοω ρεχιρχυλατιον σηιφτινγ](https://static.fdocument.org/doc/165x107/5ae59b8e7f8b9acc268c3d97/tc-atuc-04-08ppt-kompatibilittsmodus-recirculation-irc-available-on-tcatcravailable.jpg)