Correction - PNASafter birth, regulates gene expression by altering receptor con-formation and...
Transcript of Correction - PNASafter birth, regulates gene expression by altering receptor con-formation and...

Correction
NEUROSCIENCECorrection for “Liver X receptor β and thyroid hormone re-ceptor α in brain cortical layering,” by Xin-jie Tan, Xiao-tangFan, Hyun-jin Kim, Ryan Butler, Paul Webb, Margaret Warner,and Jan-Åke Gustafsson, which appeared in issue 27, July 6,2010, of Proc Natl Acad Sci USA (107:12305–12310; first pub-lished June 21, 2010; 10.1073/pnas.1006162107).The authors note that Fig. 1 appeared incorrectly. The cor-
rected figure and its legend appear below.
E6316–E6317 | PNAS | October 11, 2016 | vol. 113 | no. 41 www.pnas.org
Dow
nloa
ded
by g
uest
on
Janu
ary
16, 2
021
Dow
nloa
ded
by g
uest
on
Janu
ary
16, 2
021
Dow
nloa
ded
by g
uest
on
Janu
ary
16, 2
021
Dow
nloa
ded
by g
uest
on
Janu
ary
16, 2
021
Dow
nloa
ded
by g
uest
on
Janu
ary
16, 2
021
Dow
nloa
ded
by g
uest
on
Janu
ary
16, 2
021
Dow
nloa
ded
by g
uest
on
Janu
ary
16, 2
021
Dow
nloa
ded
by g
uest
on
Janu
ary
16, 2
021

www.pnas.org/cgi/doi/10.1073/pnas.1614988113
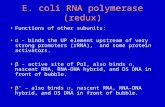
Fig. 1. Morphological alteration of embryonic and early postnatal cortex in LXRβ−/− mice. (A–I) Sagittal sections of the E15.5 neocortex and coronal sectionsof P2 and P14 stained with cresyl violet. (A and B) At E15.5, the CP is thinner and density of neurons in the IZ is higher in LXRβ−/− mice (B) than in WT controls(A). (C and D) At P2, the layers II/III in LXRβ−/− mice (D) are thinner than in WT controls (C) and the neuronal density in layers IV and V of LXRβ−/− mice (D) ishigher than that of WT controls (C). (E and F) No overall morphological difference can be observed in the cortex in LXRβ−/− mice (F) compared with WTcontrols (E) at P14. (A–F) There is no visible difference in the MZ, SVZ, and VZ at E15.5 (A and B) and layer I at P2 (C and D) or P14 (E and F) between WT andLXRβ−/− mice. (G and I) The thickness of cortical layers at E15.5 (G) (*P = 0.002 for CP; **P = 0.012 for IZ of LXRβ−/− mice compared with WT controls, byStudent’s t test), and the thickness of layers II/III at P2 and P14 (I) (*P = 0.01 at P2 for LXRβ−/− mice compared with WT controls). (H) The density of cells/0.1 mm2.In LXRβ−/− mouse brain, there is a significant increase in the IZ at E15.5 (*P = 0.001), layer IV (**P = 0.032), and V (***P = 0.008) at P2. All of the results wereexpressed as mean ± SD. Three brains were used for each genotype. (Scale bars: A–F, 50 μm.)
PNAS | October 11, 2016 | vol. 113 | no. 41 | E6317
CORR
ECTION
Dow
nloa
ded
by g
uest
on
Janu
ary
16, 2
021

Liver X receptor β and thyroid hormone receptor α inbrain cortical layeringXin-jie Tana, Xiao-tang Fanb, Hyun-jin Kima, Ryan Butlera, Paul Webbc, Margaret Warnera, and Jan-Åke Gustafssona,d,1
aCenter for Nuclear Receptors and Cell Signaling, University of Houston, Houston, TX 77204; bDepartment of Neurobiology, Third Military Medical University,Chongqing 400038, China; cMethodist Hospital Research Institute, Houston, TX 77030; and dDivision of Medical Nutrition, Department of Biosciences andNutrition, Karolinska Institute, Novum, 141 86 Stockholm, Sweden
Contributed by Jan-Åke Gustafsson, May 4, 2010 (sent for review February 27, 2010)
In the past year, two members of the nuclear receptor family, liverX receptor β (LXRβ) and thyroid hormone receptor α (TRα), havebeen found to be essential for correct migration of neurons in thedeveloping cortex in mouse embryos. TRα and LXRβ bind to iden-tical response elements on DNA and sometimes regulate the samegenes. The reason for the migration defect in the LXRβ−/− mouseand the possibility that TRα may be involved are the subjects ofthe present study. At E15.5, expression of reelin and VLDLR wassimilar but expression of apolipoprotein E receptor 2 (ApoER2) (thereelin receptor) was much lower in LXRβ−/− than in WT mice.Knockout of ApoER2 is known to lead to abnormal cortical lami-nation. Surprisingly, by postnatal day 14 (P14), no morphologicalabnormalities were detectable in the cortex of LXRβ−/− mice andApoER2 expression was much stronger than in WT controls. Thus,a postnatal mechanism leads to increase in ApoER2 expression byP14. TRα also regulates ApoER2. In both WT and LXRβ−/− mice,expression of TRα was high at postnatal day 2. By P14 it was re-duced to low levels in WT mice but was still abundantly expressedin the cortex of LXRβ−/− mice. Based on the present data we hy-pothesize that reduction in the level of ApoER2 is the reason forthe retarded migration of later-born neurons in LXRβ−/− mice butthat as thyroid hormone (TH) increases after birth the neurons dofind their correct place in the cortex.
apolipoprotein E receptor 2 | cerebral cortex | development | embryo
Liver X receptor (LXR) is a subfamily of the nuclear receptorfamily of transcription factors. The two members of this
subfamily are LXRα (1), which plays a key role in cholesterolhomeostasis and LXRβ (2), which has irreplaceable functions inthe central nervous system (3–6).We have previously shown that LXRβ regulates brain choles-
terol levels and that LXRβ expression is essential for maintenanceof motor neurons in the spinal cord and dopaminergic neurons inthe substantia nigra, suggesting that there are important roles forLXR action in brain development and, possibly, also in neuro-logical disease (7, 8). LXRβ is widely expressed in mouse brain atlater embryonic stages and is localized in the upper layers of thecerebral cortex in normal postnatal mice (9). Our analysis of braindevelopment in LXRβ−/− mice revealed smaller brain size, whichwas caused by a reduction in the number of neurons in superficialcortical layers (9). Mammalian corticogenesis involves layering ofneurons in an “inside-out” fashion, with earliest generated neuronspositioned in the deepest layers and later generated neurons mi-grating beyond previously established layers to adopt progressivelymore superficial levels (9, 10). This process is essential for corticalstructure and establishment of correct neural connections. Wehave shown that the defect in cortical development in LXRβ−/−
mice is a direct consequence of an inability of later-born neuronsto migrate to superficial layers and that this, in turn, is a conse-quence of abnormalities in vertical processes of radial glial cellsalong which migrating neurons travel (9). Thus, LXRβ playsa specific role in cortex lamination and is essential for radial mi-gration of later-generated neocortical neurons in embryonic mice.
Cortical abnormalities observed with LXRβ−/− mice are strik-ingly similar to defects produced by mutations of apolipoprotein Ereceptor 2 (ApoER2) (11), originally termed the LDL receptor-related protein CD91 (12). ApoER2 and its ligand reelin are es-sential for migration of developing cortical neurons to find theirplace in the correct layer of the cortex (11, 13). Reelin is normal inLXRβ−/− mice (9), but the fact that there are defects in neuronalmigration in the cortex raises the question of whether there maybe defects in ApoER2.Many members of the nuclear hormone receptor family play
important roles in brain development. Thyroid hormone (TH)regulates brain development and hypothyroidism can lead tomental retardation, anxiety, and even psychosis (14, 15). THsignaling is mediated by two closely related thyroid hormonereceptors (TRs), with the TRα subtype strongly expressed in thebrain (16). Like LXRs, the TRs are mostly localized in nucleiand associate with DNA in the presence and absence of theircognate ligand. TH, whose circulating levels increase sharplyafter birth, regulates gene expression by altering receptor con-formation and changing the complement of coregulators that arerecruited to TR on promoters of target genes (17–19). Recentevidence suggests that TRs can also relocate to the cytoplasm,where they may trigger rapid second messenger signaling events(20). The role of cytoplasmic TRs is not clear.LXRs bind to the same response element on DNA as TRs and
sometimes regulate the same genes (21–23). The defects incholesterol homeostasis seen in the livers of LXRα−/− mice arenot compensated for by TRs (24), but interactions of LXRs andTRs in the CNS have not been investigated.In the present study, we have analyzed the architecture of the
cerebral cortex in embryonic and neonatal LXRβ−/− mice. Re-markably, morphological abnormalities of the cortex seen atE15.5 are normalized between postnatal day 2 (P2) and postnatalday 14 (P14). We now present evidence for the role of ApoER2and TR in the abnormalities and reparation of the defects andpropose that TRα compensates for the lack of LXRβ in corticaldevelopment.
ResultsAbnormal Cortical Layers in LXRβ−/− Mice at a Late Embryonic Stageand Early Postnatal Stages. The laminated structure of the cerebralcortex was examined at later embryonic and early postnatal stagesand a comparison was made between LXRβ−/− mice and WTcontrols with cresyl violet Nissl staining. At E15.5, the cortical plate(CP) appeared thinner in the LXRβ−/− mice than in WT controls,whereas the intermediate zone (IZ) was thicker in the mutant micethan in WT controls (Fig. 1 A, B, and G). There was no overall
Author contributions: X.T., M.W., and J.-Å.G. designed research; X.T., X.F., H.K., R.B., andM.W. performed research; X.T., P.W., M.W., and J.-Å.G. analyzed data; and X.T., M.W.,and J.-Å.G. wrote the paper.
The authors declare no conflict of interest.1To whom correspondence should be addressed. E-mail: [email protected].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006162107/-/DCSupplemental.
www.pnas.org/cgi/doi/10.1073/pnas.1006162107 PNAS | July 6, 2010 | vol. 107 | no. 27 | 12305–12310
NEU
ROSC
IENCE

difference in the appearance or thickness of the marginal zone(MZ), subventricular zone (SVZ), or ventricular zone (VZ).However, the density of neurons occupying the IZ of LXRβ−/−mice was higher than in WT controls (Fig. 1 A, B, and H). Thisindicates that migration of many neurons was arrested at the IZ.To determine the consequences of the halt in neuronal mi-
gration during embryogenesis on newborn mice, the cortex wasexamined at an early (P2) and a later postnatal stage (P14). AtP2, the layers II/III occupied by the later-born neurons wasthinner in LXRβ−/− mice and the neuronal density in layers IVand V was greater than that of WT controls (Fig. 1 C, D, H, andI). Surprisingly, however, no overall morphological differences inthe cortex were observed between WT and LXRβ−/− mice at P14(Fig. 1 E, F, H, and I). There were no visible differences in layer I(Fig. 1 C and D) and the hippocampus at P2 (Fig. S1 A and B) orP14 (Fig. S1 C and D) between WT and LXRβ−/− mice. Thus,the early defects in cerebral cortex architecture are corrected inthe postnatal period.
Important Role of LXRβ for Early and Later-Generated NeuronalMigration. To know which stage of neuronal migration wasinfluenced by LXRβ, the transcription factors T box brain 1(TBR1) and Brn2 were used as early-generated and later-generated neuronal labels, respectively (9, 10). At E15.5, TBR1was highly expressed at the lower CP. Lower levels were observedat the IZ/SVZ, and it was not detectable in the VZ (Fig. 2 A andB). More TBR1-positive neurons were located at IZ in LXRβ−/−mice than WT controls (Fig. 2 A, B, and S). No statistical differ-ence for TBR1-positive neuronal density was observed in the CPbetween LXRβ−/− mice and WT controls at E15.5 (Fig. 2 A, B,and S). A few focal CP dysplasias were observed in LXRβ−/−miceat E15.5 (Fig.2 B), but at P2 (Fig. 2 C and D) and P14 (Fig. 2 Eand F) the focal CP dysplasias were no longer present. At P2 in theLXRβ−/− mouse brain, the density of TBR1-positive neurons inlayer VI was higher than that in WTmice (Fig. 2 C,D, and S). Nosuch difference was detectable at P14 (Fig. 2 E, F, and S). AtE15.5, there were fewer Brn2-positive neurons located in the su-perficial CP of LXRβ−/− mouse brain than in the WT controls(Fig. 2 G, H, and T). These differences indicate that the later-generated neuronsmigrate to the superficial CP to form the futurelayers IV, III, and II according to the right “inside-out” sequencein WT controls (9) but that in LXRβ−/− mice, Brn2-positiveneurons could not migrate to the superficial CP. At P2, there wasno difference between WT and LXRβ−/− mice in the density ofBrn2-positive neurons in layers II/III measured as cells/0.1 mm2
(Fig. 2 I, J, and T). However, the total number of Brn2-positiveneurons in layers II/III of LXRβ−/− mice was lower than in WTcontrols because this layer was thinner in LXRβ−/− mice (Fig. 2 Iand J). At P14 there was no visible difference between LXRβ−/−mice and WT controls in the thickness of the Brn2-positive layersII/III and density of Brn2-positive neurons in layers II/III (Fig. 2K,L, and T) underscoring the idea that there is full recovery ofcortical architecture between P2 and P14.Immunohistochemical staining with Neuron-specific class III
beta-tubulin (TuJ1), a marker of immature neurons and NeuronalNuclei (NeuN), a marker of postmitotic neurons, were used in thisstudy (25). TuJ1 immunofluorescent staining showed fewer posi-tive neurons in the CP in LXRβ−/−mice than WT controls at E15.5(Fig. 2 M and N). At P2, most of the NeuN-positive neurons werelocated at the lower parts of layers II/III but more neuronsremained at the layers IV and V in the LXRβ−/−mice than theWTcontrols (Fig. 2 O, P, and U). Many immature neurons occupyingthe superficial parts of layers II/III at P2 were NeuN-negative (Fig.2 O and P), and these neurons became mature at P14 in LXRβ−/−mouse cortex and WT controls (Fig. 2 Q and R) so that there wasno visible difference in NeuN distribution at P14 between LXRβ−/−mouse cortex and WT controls (Fig. 2 Q, R, and U).
Fig. 1. Morphological alteration of embryonic and early postnatal cortex inLXRβ−/− mice. (A–I) Sagittal sections of the E15.5 neocortex and coronalsections of P2 and P14 stained with cresyl violet. (A and B) At E15.5, the CP isthinner and density of neurons in the IZ is higher in LXRβ−/− mice (B) than inWT controls (A). (C and D) At P2, the layers II/III in LXRβ−/− mice (D) arethinner than in WT controls (C) and the neuronal density in layers IV and Vof LXRβ−/− mice (D) is higher than that of WT controls (C). (E and F) Nooverall morphological difference can be observed in the cortex in LXRβ−/−
mice (F) compared with WT controls (E) at P14. (A–F) There is no visibledifference in the MZ, SVZ, and VZ at E15.5 (A and B) and layer I at P2 (C andD) or P14 (E and F) between WT and LXRβ−/− mice. (G and I) The thickness ofcortical layers at E15.5 (G) (*P = 0.002 for CP; **P = 0.012 for IZ of LXRβ−/−
mice compared with WT controls, by Student’s t test), and the thickness oflayers II/III at P2 and P14 (I) (*P = 0.01 at P2 for LXRβ−/− mice compared withWT controls). (H) The density of cells/0.1 mm2. In LXRβ−/− mouse brain, thereis a significant increase in the IZ at E15.5 (*P = 0.001), layer IV (**P = 0.032),and V (***P = 0.008) at P2. All of the results were expressed as mean ± SD.Three brains were used for each genotype. (Scale bars: A–F, 50 μm.)
12306 | www.pnas.org/cgi/doi/10.1073/pnas.1006162107 Tan et al.

Abnormal Expression of ApoER2 and Abnormal Scaffold of Radial GliaFibers in E15.5 Neocortex of LXRβ−/− Mice. There was no visibledifference in the reelin expression in MZ between WT andLXRβ−/− mice at E15.5 (Fig. 3 A and B). Therefore, we exam-
ined whether the abnormalities observed could be caused bya defect in the reelin receptors, ApoER2 and VLDLR (26). In theWTmouse cortex at E15.5, ApoER2 was distributed mostly in theMZ, lowerCP, and subplate (SP) (Fig. 3C,E, andM). However, in
Fig. 2. Distribution of cortical layer markers TBR1 and Brn2 and neuronal specific markers. (A and B) TBR1 is located at the CP and IZ/SVZ, especially at thelower CP, but is absent in the VZ in WT controls (A) and LXRβ−/− mice (B) at E15.5. (B) A few focal CP dysplasias can be found in LXRβ−/− mice. (A–F) In theLXRβ−/− mouse brain, there is a significant increase in TBR1-positive neuronal density in the IZ at E15.5 (B) and in layer VI at P2 (D) compared with WT controls(A and C), but there is no difference at P14 (E and F). (G–L) Brn2-positive neurons locating in the superficial CP at E15.5 (H) and layers II/III at P2 (J) of LXRβ−/−
mouse brain are fewer than in WT controls (G and I), but there is no difference at P14 (K and L). (M and N) TuJ1 staining shows that there are fewer positiveneurons in the CP in LXRβ−/− mice (N) than in WT controls (M) at E15.5. (O and P) Most of the NeuN-positive neurons are located in the lower part of layers II/IIIat P2 but more NeuN-positive neurons stay at the layers IV and V in LXRβ−/− mice (P) than in WT controls (O). (Q and R) NeuN staining shows no overalldifference between LXRβ−/− and WT cortex at P14. (S) The density of TBR1-positive cells in the CP and IZ at E15.5 (n = 3; *P = 0.004 for LXRβ−/− mice comparedwith WT controls) and layer VI at P2 (n = 3; **P = 0.006 for LXRβ−/− mice compared with WT controls) and P14 is shown. (T) The density of Brn2-positiveneurons in the CP at E15.5 and layers II/III at P2 and P14 (n = 3; *, P = 0.007 for LXRβ−/− mice compared with WT controls). (U) The density of NeuN-positiveneurons in cortical layers at P2 and P14. In the LXRβ−/− mouse brain there is a significant increase in NeuN-positive neuronal density in the layers IV and V at P2(n = 3; *P = 0.008; **P = 0.001). (Scale bars: A, B, G, H, M, N, 100 μm; C–F, I–L, O–R, 50 μm.)
Tan et al. PNAS | July 6, 2010 | vol. 107 | no. 27 | 12307
NEU
ROSC
IENCE

the LXRβ−/− mouse brain there was remarkably weak staining inthe SP (Fig. 3 D, F, and Q). ApoER2 was expressed on the neu-ronal projections andmembrane at the embryonic stage (Fig. 3M–
T) and only on the neuronal membrane and cytoplasm at P14 (Fig.3 I and J). At P2, the ApoER2 staining was not detectable, but atP14, ApoER2 expression in LXRβ−/− mice was very strong,whereas it was barely detectable in layers II/III in WT controls(Fig. 3 I and J).To examine whether higher expression of ApoER2 in the
LXRβ−/− mouse brain at P14 is due to a difference in degradationof the protein, we examined expression of the protein normallyinvolved in its degradation, i.e., PSCK9 (27). Immunohistochem-ical staining with a PCSK9 antibody showed no staining at E15.5,but at P14 expression of PSCK9 in LXRβ−/− mice was strongerthan in WT controls (Fig. 3 K and L), indicating that decreaseddegradation by PCSK9 is not responsible for the accumulation ofApoER2 in the LXRβ−/− mouse brain in the postnatal period.At E15.5, the scaffolds of radial glial fibers, detected with
antibodies against Rat401 (28) in WT controls, were more intact
and more processes were distributed throughout the CP than inLXRβ−/− mouse cortex (Fig. 3 G and H). VLDLR, a stop signalfor neuronal migration, was examined, but there was no stainingat E15.5 or P2.
Up-Regulation of Thyroid Hormone Receptor α. The focal CP dys-plasia in LXRβ−/− mice observed at E15.5 disappeared afterbirth, and no morphological differences in the cortex could bediscerned at P14 between WT and LXRβ−/− mice regarding theNissl and layer-marker staining. Thus, some mechanism mustcompensate for the absence of LXRβ in the time between P2 andP14. Because LXRs and TRs sometimes regulate similar genes,we tested the possibility that alterations in TR signaling pathwayscould play a compensatory role and repair the neuronal deficitcaused by loss of LXRβ (21–23). At P2, there was strong nuclearstaining for TRα in layers II/III (Fig. 4 A and B) and the density
Fig. 3. Abnormal expression of ApoER2 and abnormal scaffold of radial gliafibers in E15.5 neocortex of LXRβ−/− mice. (A and B) There is no visible dif-ference in the reelin expression (Red) in the MZ between WT and LXRβ−/−
mice at E15.5. Nuclei were counterstained with DAPI (Blue). (C, E, and M)ApoER2 is distributed mostly in the MZ, lower CP and SP in WT controls atE15.5. (D, F, and Q) ApoER2 is decreased sharply in the SP in LXRβ−/− mousebrain. (E and F) Higher-power views of the boxed areas in C and D. (I, J, andM–T) ApoER2 is expressed on neuronal projections and membrane duringE15.5 (M–T) but only expressed on the neuronal membrane and cytoplasmat P14 (I and J). (I–L) The expression of ApoER2 (J) and PCSK9 (L) appearsstronger in LXRβ−/− mouse brain than in WT controls (I and K) at P14. (G andH) The scaffold of radial glial fibers is more intact and more processes aredistributed in the CP in WT controls (G) than in LXRβ−/− mice (H) at E15.5.(Scale bars: A, B, and E–T, 100 μm; C, D, 50 μm.)
Fig. 4. Expression of TRα and proliferation in LXRβ−/−mouse cortex. (A and B)At P2, layers II/III are occupied by TRα-positive cells and all of the cells havenuclear staining inWT controls (A) and LXRβ−/−mice (B). (C andD) By contrast,at P14, TRα-positive cells distributing throughout the LXRβ−/− mouse cortex(D) are far more abundant than in WT controls (C). (A and D Insets) Highermagnification image of area in box; note strictly nuclear staining in A Insetand cytoplasmic staining in B Inset. (E–H) At P2, more Ki67-positive cells areobserved in the WT control cortex (E and G) than in LXRβ−/− mice (F and H).(G andH) Higher-power views of the boxed areas in E and F. (K) The density ofTRα-positive cells in layers II/III in LXRβ−/−mice at P2 (n=3; *P=0.002 comparedwithWT controls) and P14 (n = 3; **P = 0.005 compared withWT controls). (L)The density of Ki67-positive cells in the cortex at P2 and P14. In the LXRβ−/−
mouse cortex the Ki67-positive neuronal density is less than in WT controls atP2 (n = 3; *P = 0.016) but no difference is seen at P14. (Scale bars: A–D, G, andH, 100 μm; E and F, 50 μm.)
12308 | www.pnas.org/cgi/doi/10.1073/pnas.1006162107 Tan et al.

of TRα-positive cells in WT controls was higher than in LXRβ−/−mice at P2 (Fig. 4 K). However, at P14, there were far moreTRα-positive cells distributed throughout the LXRβ−/− mousecortex than in the WT controls (Fig.4 C, D and K). In contrast toP2, however, almost all of the TRα-positive cells at day 14exhibited cytoplasmic staining (Fig. 4 A and D Insets). TRα isimplicated in neuroblast proliferation (29) so we tested thepossibility that TRα-dependent proliferation accounts for re-covery of cortical morphology. Ki67 is a proliferation markerthat is expressed by proliferating cells in all phases of the activecell cycle (G1, S, G2, and M phase) but not in resting (G0) cells(30). At P2, more proliferation was observed in the WT controlcortex than in the LXRβ−/− mice (Fig. 4 E–H and L). At P14 nodifference in Ki67 expression was detectable between LXRβ−/−mice and WT controls. Thus, changes in TRα expression andsubcellular localization are not related to changes in corticalneuronal proliferation.
DiscussionLXRβ is expressed ubiquitously in the brain and appears in theneurons as early as E14.5 (9). Knockout of LXRβ gives rise toabnormal cortex lamination because of the retardation in themigration of later-born neurons. The inability to migrate appearsto be due to truncation of the vertical processes of radial glia (9)upon which the migrating neurons depend for their direction.In the present study, we demonstrate that in LXRβ−/− mice,
the cortical development is aberrant at later embryonic and earlypostnatal stage but recovers at later postnatal stages. At E15.5,the cortical plate was thinner in LXRβ−/− mice brains than in WTmice and there was an accumulation of neurons at the IZ. At P2,the cortex developed from the cortical plate was still thinner inLXRβ−/− mice with fewer neurons in layers II/III and in contrast,more neurons at layers IV and V. Surprisingly however, thecortex appeared normal by P14. This normalization suggestedthat those neurons that were arrested at layers IV and V at P2 inLXRβ−/− mice migrated to the superficial layers later. Althoughloss of LXRβ arrested cortical neuronal migration, the laminationand morphology of the hippocampus were normal at P2 and P14.Analysis of early and late-born neuronal markers supports
the idea that there is an early deficit in neuronal migration inLXRβ−/− mice but that the brain compensates for this deficit be-tween P2 and P14. At E15.5, there were fewer TBR1-positiveneurons (early-born neurons) (9, 10) in CP in LXRβ−/− mice, al-though the cell density was not different. However in the IZ, as wasevident with Nissl staining, there were more TBR1-positive neu-rons in LXRβ−/−mice than inWT controls. At P14, no differencesin the distribution of TBR1-positive neurons were observed be-tween WT and LXRβ−/− mice. In addition, there were a few focalcortical plate dysplasias in LXRβ−/− mice at E15.5, but none weredetectable after birth. These results suggest that some neuronsrecovered the ability to migrate after birth. Brn2 is a marker oflater-born neurons destined to superficial CP to form the futurecortical layers II/III (9, 10). At E15.5 and P2, there were fewerBrn2-positive neurons in CP in the LXRβ−/− mice than in WTcontrols. However, at P14 there were no differences in the numberor location of Brn2-positive neurons between WT and LXRβ−/−mice. Clearly, as described previously (9), migration of later-bornneurons was arrested when LXRβ was not expressed but somemechanism compensated for this deficit between P2 and P14.Immunofluorescent staining showed that at E15.5, there were
fewer TuJ1-positive neurons (25) in the CP of the LXRβ−/− mice.This is consistent with the Nissl and TBR1 staining results. At P2,most of the NeuN-positive neurons in WTmice were located at thelower parts of layers II/III but more neurons remained in the layersIVandV in theLXRβ−/−mice than in theWTcontrols.Almost all ofthe superficial neurons in layers II/III of LXRβ−/−mice were NeuN-negative indicating that these neurons were latest-born and imma-ture. No difference of NeuN-positive (mature neurons) staining was
observed between LXRβ−/−mice andWT controls at P14. Thus theimmature neurons located in the superficial layers in both mouselines at P2 becamemature at P14, and by this time, the abnormalitiesin LXRβ−/−mice had normalized.We propose that the deficit in neuronal migration in LXRβ−/−
mice is related to reduced expression of ApoER2, the reelinreceptor. Reelin has functions in the developing brain (31–35).In cortical development, reelin is crucial for correct positioningof radially migrating neuronal precursors via its binding toApoER2 and VLDLR on neuronal precursors (11, 36, 37). InApoER2 mutants, many later-born neurons fail to migrate totheir destinations in superficial cortical layers, and some neuronsinvade the marginal zone in VLDLR mutants (10). Thus, the tworeelin receptors, VLDLR and ApoER2, play divergent roles,with VLDLR mediating a stop signal for migrating neurons andApoER2 as an essential signal for the migration of later-bornneurons (10). In the present study, we did not find any abnor-malities of the number of Cajal-Retzius neurons nor the intensityof their immunostaining for reelin in LXRβ−/− mice (9). How-ever, expression of ApoER2 was different in LXRβ−/− mice.Later-born neurons destined to superficial cortical layersstrongly express both LXRβ (9) and ApoER2 (10), suggestingthat ApoER2 may be regulated by LXRβ. In the WT mousecortex at E15.5, ApoER2 was distributed mostly in the MZ,lower CP, and subplate (SP) (10). In the LXRβ−/− mouse brain,there was weak staining for ApoER2 in the SP. Costaining ofTuJ1 and ApoER2 showed that ApoER2 was expressed morestrongly on the neuronal projections and membrane duringembryonic development.At P14, ApoER2 expression in LXRβ−/− mice was very strong
in layers II/III, whereas it was barely detectable in WT controls.Therefore, ApoER2 appears to have a different regulation modeand role during the embryonic and postnatal stages. To examinewhether the higher expression of ApoER2 in the LXRβ−/− brainat P14 is due to a difference in degradation of the protein, theenzyme—PSCK9—responsible for degradation of ApoER2 (27,38, 39) was examined. Immunohistochemical staining for PCSK9showed that decreased degradation was not a likely cause for theaccumulation of ApoER2 at P14.LXRs bind to the same response element on DNA as does TR
and regulates many of the same genes (21–23). Although TRdoes not compensate for the function of LXRs in LXR knockoutmice in cholesterol homeostasis (24), it is possible that TR cancompensate for some of the defects due to loss of LXRβ in theCNS. TRα plays a key role in postnatal development where itpresumably mediates most of the thyroid hormone effects. TRβis detected in few areas of the brain (16, 40, 41). We found strongnuclear staining for TRα in WT and LXRβ−/− mice at P2.However, at P14, there were far more TRα-positive cells dis-tributed throughout the LXRβ−/− mouse cortex than in the WTcontrols. This finding raises the possibility that increased ex-pression of TRαmay compensate for loss of LXRβ and stimulatethe retarded neurons to migrate to their correct position. It isnoteworthy that almost all of the TRα staining was cytoplasmicat P14. This is in marked contrast to P2, where TRα was strictlynuclear. From Ki67 staining, it appears that there was no in-crease in neuronal proliferation in the postnatal period inLXRβ−/− mice. Therefore, a role of TR in normalizing the cortexis in migration, not in proliferation.We suggest that there may be two possible effects of TRα on
LXR signaling in cortical development. First, unliganded TRssuppress gene transcription. Thus, migration of TRα from a nu-clear to cytoplasmic location could derepress ApoER2 geneexpression by removing inhibitory influences at possible TRE/LXRE elements (42). Alternatively, plasma levels of TH increasesharply during neonatal and postnatal periods (42–44) and in thepresence of its ligand, TR leaves the nucleus and is cytoplasmic.
Tan et al. PNAS | July 6, 2010 | vol. 107 | no. 27 | 12309
NEU
ROSC
IENCE

Cytoplasmic TR may modulate cell behavior and gene expressionby triggering second messenger pathways (17–19).We conclude that reduction in the level of ApoER2 is the likely
reason for the retarded migration of later born neurons in LXRβ−/−embryonic mice but that postnatal changes in TR signaling path-ways permit neurons to find their correct place in the cortex (45).
Materials and MethodsThegeneration of LXRβ−/−micehasbeendescribed (3).Heterozygousmicewereused for breeding. The day of vaginal plug detection was designated as E0.5.Embryonic andP2andP14micebrainswereobtainedasdescribed in SIMaterials
and Methods. Immunohistochemistry and immunofluorescence staining wereperformed as previously described (7, 9). Detailed procedures are described inSI Materials and Methods. For layer thickness measuring and cell counting,MicroSuite Basic Edition and Image Pro Plus 6.0 were used, respectively. Thenumber of mice in each experiment was at least three per genotype. Data arepresented as mean ± SD. The statistical significance of differences betweenLXRβ−/− and control samples was assessed by using Student’s t test.
ACKNOWLEDGMENTS. This work was supported by a grant from theSwedish Science Council and the European Integrated Project on NuclearReceptors, Chemicals as contaminants in the food chain: an NoE for research,risk assessment and education (CASCADE).
1. Apfel R, et al. (1994) A novel orphan receptor specific for a subset of thyroidhormone-responsive elements and its interaction with the retinoid/thyroid hormonereceptor subfamily. Mol Cell Biol 14:7025–7035.
2. Teboul M, et al. (1995) OR-1, a member of the nuclear receptor superfamily thatinteracts with the 9-cis-retinoic acid receptor. Proc Natl Acad Sci USA 92:2096–2100.
3. Alberti S, et al. (2001) Hepatic cholesterol metabolism and resistance to dietarycholesterol in LXRbeta-deficient mice. J Clin Invest 107:565–573.
4. Robertson KM, et al. (2005) The liver X receptor-β is essential for maintainingcholesterol homeostasis in the testis. Endocrinology 146:2519–2530.
5. Steffensen KR, et al. (2004) Genome-wide expression profiling; a panel of mousetissues discloses novel biological functions of liver X receptors in adrenals. J MolEndocrinol 33:609–622.
6. Wang L, et al. (2002) Liver X receptors in the central nervous system: From lipidhomeostasis to neuronal degeneration. Proc Natl Acad Sci USA 99:13878–13883.
7. Kim HJ, et al. (2008) Liver X receptor beta (LXRbeta): A link between beta-sitosteroland amyotrophic lateral sclerosis-Parkinson’s dementia. Proc Natl Acad Sci USA 105:2094–2099.
8. Andersson S, Gustafsson N, Warner M, Gustafsson JA (2005) Inactivation of liver Xreceptor beta leads to adult-onset motor neuron degeneration in male mice. ProcNatl Acad Sci USA 102:3857–3862.
9. Fan X, Kim HJ, Bouton D, Warner M, Gustafsson JA (2008) Expression of liver Xreceptor beta is essential for formation of superficial cortical layers and migration oflater-born neurons. Proc Natl Acad Sci USA 105:13445–13450.
10. Hack I, et al. (2007) Divergent roles of ApoER2 and Vldlr in the migration of corticalneurons. Development 134:3883–3891.
11. Trommsdorff M, et al. (1999) Reeler/Disabled-like disruption of neuronal migration inknockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97:689–701.
12. Stöhr J, Schindler G, Rothe G, Schmitz G (1998) Enhanced upregulation of the Fcgamma receptor IIIa (CD16a) during in vitro differentiation of ApoE4/4 monocytes.Arterioscler Thromb Vasc Biol 18:1424–1432.
13. Lambert de Rouvroit C, Goffinet AM (1998) The reeler mouse as a model of braindevelopment. Adv Anat Embryol Cell Biol 150:1–106.
14. Zamoner A, Heimfarth L, Pessoa-Pureur R (2008) Congenital hypothyroidism isassociated with intermediate filament misregulation, glutamate transporters down-regulation and MAPK activation in developing rat brain. Neurotoxicology 29:1092–1099.
15. Nayak B, Hodak SP (2007) Hyperthyroidism. Endocrinol Metab Clin North Am 36:617–656, v.
16. Bradley DJ, Young WS, 3rd, Weinberger C (1989) Differential expression of alpha andbeta thyroid hormone receptor genes in rat brain and pituitary. Proc Natl Acad SciUSA 86:7250–7254.
17. Yen PM, Wilcox EC, Hayashi Y, Refetoff S, Chin WW (1995) Studies on the repressionof basal transcription (silencing) by artificial and natural human thyroid hormonereceptor-beta mutants. Endocrinology 136:2845–2851.
18. Matsuda H, Paul BD, Choi CY, Shi YB (2007) Contrasting effects of two alternativesplicing forms of coactivator-associated arginine methyltransferase 1 on thyroidhormone receptor-mediated transcription in Xenopus laevis. Mol Endocrinol 21:1082–1094.
19. Paul BD, Fu L, Buchholz DR, Shi YB (2005) Coactivator recruitment is essential forliganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol CellBiol 25:5712–5724.
20. Guigon CJ, Cheng SY (2009) Novel non-genomic signaling of thyroid hormonereceptors in thyroid carcinogenesis. Mol Cell Endocrinol 308:63–69.
21. Enmark E, Gustafsson JA (2001) Comparing nuclear receptors in worms, flies andhumans. Trends Pharmacol Sci 22:611–615.
22. Berkenstam A, Färnegårdh M, Gustafsson JA (2004) Convergence of lipid homeostasisthrough liver X and thyroid hormone receptors. Mech Ageing Dev 125:707–717.
23. Quack M, Frank C, Carlberg C (2002) Differential nuclear receptor signalling fromDR4-type response elements. J Cell Biochem 86:601–612.
24. Peet DJ, Janowski BA, Mangelsdorf DJ (1998) The LXRs: A new class of oxysterolreceptors. Curr Opin Genet Dev 8:571–575.
25. Kornack DR, Rakic P (2001) Cell proliferation without neurogenesis in adult primateneocortex. Science 294:2127–2130.
26. Huang Z (2009) Molecular regulation of neuronal migration during neocorticaldevelopment. Mol Cell Neurosci 42:11–22.
27. Poirier S, et al. (2008) The proprotein convertase PCSK9 induces the degradation oflow density lipoprotein receptor (LDLR) and its closest family members VLDLR andApoER2. J Biol Chem 283:2363–2372.
28. Rosen GD, Sherman GF, Galaburda AM (1994) Radial glia in the neocortex of adultrats: Effects of neonatal brain injury. Brain Res Dev Brain Res 82:127–135.
29. Lezoualc’h F, et al. (1995) Inhibition of neurogenic precursor proliferation byantisense alpha thyroid hormone receptor oligonucleotides. J Biol Chem 270:12100–12108.
30. Gerdes J, et al. (1991) Immunobiochemical andmolecular biologic characterizationof thecell proliferation-associated nuclear antigen that is defined by monoclonal antibodyKi-67. Am J Pathol 138:867–873.
31. Andrade N, et al. (2007) ApoER2/VLDL receptor and Dab1 in the rostral migratorystream function in postnatal neuronal migration independently of Reelin. Proc NatlAcad Sci USA 104:8508–8513.
32. Dulabon L, et al. (2000) Reelin binds alpha3beta1 integrin and inhibits neuronalmigration. Neuron 27:33–44.
33. Gilmore EC, Herrup K (2000) Cortical development: Receiving reelin. Curr Biol 10:R162–R166.
34. Heins N, et al. (2002) Glial cells generate neurons: The role of the transcription factorPax6. Nat Neurosci 5:308–315.
35. SanadaK, GuptaA, Tsai LH (2004) Disabled-1-regulated adhesion ofmigrating neuronsto radial glial fiber contributes to neuronal positioning during early corticogenesis.Neuron 42:197–211.
36. D’Arcangelo G, et al. (1999) Reelin is a ligand for lipoprotein receptors. Neuron 24:471–479.
37. Hiesberger T, et al. (1999) Direct binding of Reelin to VLDL receptor and ApoEreceptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tauphosphorylation. Neuron 24:481–489.
38. Maxwell KN, Breslow JL (2004) Adenoviral-mediated expression of Pcsk9 in miceresults in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad SciUSA 101:7100–7105.
39. Rashid S, et al. (2005) Decreased plasma cholesterol and hypersensitivity to statins inmice lacking Pcsk9. Proc Natl Acad Sci USA 102:5374–5379.
40. Rivas M, Naranjo JR (2007) Thyroid hormones, learning and memory. Genes BrainBehav 6(Suppl 1):40–44.
41. Mellström B, Naranjo JR, Santos A, Gonzalez AM, Bernal J (1991) Independentexpression of the alpha and beta c-erbA genes in developing rat brain. MolEndocrinol 5:1339–1350.
42. Pathak A, Sinha RA, Mohan V, Mitra K, Godbole MM (2010) Maternal thyroidhormone before the onset of fetal thyroid function regulates reelin and downstreamsignaling cascade affecting neocortical neuronal migration. Cereb Cortex, 10.1093/cercor/bhq052.
43. Bernal J, Guadaño-Ferraz A, Morte B (2003) Perspectives in the study of thyroidhormone action on brain development and function. Thyroid 13:1005–1012.
44. Porterfield SP, Hendrich CE (1993) The role of thyroid hormones in prenatal andneonatal neurological development—current perspectives. Endocr Rev 14:94–106.
45. Williams GR (2008) Neurodevelopmental and neurophysiological actions of thyroidhormone. J Neuroendocrinol 20:784–794.
12310 | www.pnas.org/cgi/doi/10.1073/pnas.1006162107 Tan et al.

![Exploring the Involvement of NLRP3 and IL-1β in …downloads.hindawi.com/journals/mi/2019/2363460.pdfhand osteoarthritis [26] were recruited in the Rheumatol-ogy Unit of Siena Hospital](https://static.fdocument.org/doc/165x107/5f93c90a1258491ec9221a4a/exploring-the-involvement-of-nlrp3-and-il-1-in-hand-osteoarthritis-26-were-recruited.jpg)