CHEM 6352 Organic Reactions & Synthesis Simple …may.chem.uh.edu/teach-files/Simple Retrons.pdfCHEM...
Transcript of CHEM 6352 Organic Reactions & Synthesis Simple …may.chem.uh.edu/teach-files/Simple Retrons.pdfCHEM...

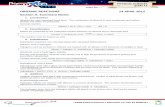
CHEM 6352 Organic Reactions & Synthesis Simple Retrons
(Α) α-Substituted Carbonyls: Enolate Alkylation
(B) ß-Hydroxy Carbonyls: Enolate Aldol
(C) ß-Alkyl Carbonyls: Cuprate Additions
(D) Homoallylic Alcohol: Allyl Addition to Carbonyl
X=SiR3, BR2, SnR3
[2,3] Wittig Reaction
(E) 1,2-Diol: Olefin Dihydroxylation
Pinacol Reaction
Epoxide Hydrolysis
X
O
R1
R2
X
O
R1 + R2-X
X
O
R1X
O
R1+R2
OH
H R2
O
O
R
O
+LiCuCN
R
R2
OH
R1
X +H R2
O
R2
OH
R1
OR2 R1
OH
OH
OH
OH
H
O
+H
O
OH
OH
O

(F) 1,3-Diol (syn): Allylic Epoxidation and Reductive Opening
ß-Hydroxy Carbonyl Reduction
1,3-Diol (anti): ß-Hydroxy Carbonyl Reduction
(G) cis-Olefins:
Wittig Olefination
Lindlar Reduction
trans-Olefins: Julia Olefination
Horner Wadsworth Emmons Reaction (R1 is stabilizing/EWG)
Birch Reduction of Alkynes
Grubbs Olefin Metathesis
OH OH OHO
OH
OH OH O OH+ NaBH4 or Zn(BH4)2
OH OH O OH+ Na(OAc)3BH
R1 R2+
H R2
O
RCHI2
R1 R2+
R1
PPh3
H R2
O
R1 R2R1 R2
R1
R2 R1 SO2Ar +H R2
O
THEN Na(Hg)
R1R2 +
R1
PO(OR)3
H R2
O
R1
R2 R1 R2
R1
R2R1
R2+

(H) syn-2-Substituted Alcohols: Directed Allylic Reduction
trans-2-Substituted Alcohols: Directed Allylic Reduction
(I) δ ,ε-Unsaturated Carbonyl: Anionic Oxy-Cope
(J) γ ,δ-Unsaturated Carbonyl: Claisen Reaction
(K) α-ß-Unsaturated Carbonyl: Aldol Condensation
(L) Cyclohexene: Diels-Alder Reaction
R2
OH
R1
R2
OH
R1
R2
OH
R1
R2
OH
R1
X
OOH
X
O Cl
BnOO Cl
OBn
X
O
O O
H
OMe
OOH OTMS O
OMe+