Cephalosporins & other β lactam antibiotics & cell wall destructors
-
Upload
farazajaved -
Category
Health & Medicine
-
view
556 -
download
1
description
Transcript of Cephalosporins & other β lactam antibiotics & cell wall destructors

Cephalosporins & other β-Lactam Antibiotics.Cell Wall Destructors.
Faraza JavedMphil Pharmacology

β- Lactam Antibiotics
β-lactam antibiotics, inhibit bacterial growth by interfering with bacterial cell wall synthesis.
The β-lactam antibiotics may be further sub-divided into two categories, namely:
PenicillinCephalosporin

Cephalosporins
Cephalosporins are similar to Penicillins, but more stable to many bacterial β lactamases and therefore have a broader spectrum of activity.

HistoryThe 1st source of Cephalosporins,
Cephalosporium acremonium(Fungus), was isolated in 1948 by Giuseppe from the sea near a sewer outlet of the Sardinian Coast.
The crude filtrates from this fungus were found to inhibit the in vitro growth of β- lactamase producing S. aureus and to cure the Staphylococcal infections and typhoid fever in humans.

Subsequently, Abraham and his colleagues identified three distinct antibiotics from the culture fluid of fungus.
These antibiotics were named Cephalosporin N and C (which were chemically related to penicillin) and Cephalosporin P, a steroid antibiotic that resembles Fusidic Acid.

Chemistry
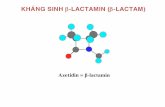
The nucleus of Cephalosporins, 7-aminocephalosporanic acid bears a close resemblance to 6-aminopencillanic acid.
The core of the basic cephalosporin molecule consists of a two ring system which includes a β-lactam ring condensed with dihydrothiazine ring. The core itself can also be referred to as 7-aminocephalosporanic acid which can be derived by hydrolysis from the natural compound Cephalosporin C.


Chemical compounds containing this core are relatively stable to acid hydrolysis and tolerance to β-lactamases. Cephalosporin C contains a side-chain which is derived from D-aminoadipic acid.

Modification of side chains on the relevant positions has been used to create a whole new class of cephalosporin antibiotics.
Modification of side-chains at position 7 of the lactam ring seems to affect the antibacterial activity while position 3 of the dihydrothiazine ring alters pharmacokinetic properties and receptor binding affinity.

Mechanism of Action
Cephalosporins exert bactericidal effect in manner similar to that of Penicillins.
Binding to specific PBPSInhibition of cell wall synthesis by
inhibiting transpeptidation of Peptidoglycan
Activation of Autolytic enzymes Autolysins or Murein Hydrolases

Classification
Cephalosporins can be classified into four major groups or generations, depending mainly on the spectrum of antimicrobial activity.
Recently, Fifth generation cephalosporins were developed in the lab to specifically target against resistant strains of bacteria particularly Methicillin Resistance Staphlococcus Aureus (MRSA).

1st Generation Cephalosporins
The agents included in this group have good activity against gram-positive cocci, such as pneumococci, streptococci and staphylococci but not active against methicillin resistant strains of staphylococci, and relatively modest activity against gram-negative microorganisms (E.Coli and Klebsiella pnumoniae).

1st generation Cephalosporins include:Cefazolin (IV/IM)Cefadroxil (PO)Cephalexin (PO)Cephalothin (IV/IM)Cephapirin (IV/IM)Cephradine (PO)

2nd Generation Cephalosporins
These compounds show modest activity against gram-positive bacteria (less active than 1st generation drugs) and display greater activity against gram-negative microorganisms including Haemophilus influenza, some Enterobacter aerogenes and Neisseria Species.

In comparison to 1st generation, they have some what increased activity against gram-negative bacteria but this activity is much less than the activity of 3rd generation compounds.

The drugs included in this class are:Cefaclor (PO)Cefamandole (IV/IM)Cefonicid (IM/IV)Cefuroxime (IV/IM/PO)Cefprozil (PO)Loracarbef (PO)Ceforanide (IM/IV)

3rd Generation Cephalosporins
Though greatly inferior to 1st generation cephalosporins in regard to their activity against gram-positive cocci, the 3rd generation cephalosporins exhibit much more activity against gram-negative bacilli, most other enteric organisms and β- lactamase producing strains of Haemophilus and Neisseria.

Drugs of this group have superiority over the other two generation in having ability to reach CNS (cross BBB). They include:
Cefoperazone (IV/IM)Cefotaxime (IV/IM)Ceftriaxone (IV/IM)Cefixime (PO)Ceftazidime (IV/IM)Moxalactam (IM/IV)

4th Generation Cephalosporins
They have an extended spectrum of activity as compared to the 3rd generation and have increased stability from hydrolysis by plasmid and chromosomally mediated β- lactamases.

Aerobic gram-negative bacilli resistant to 3rd generation cephalosporins can be succesfully treated with 4th generation drugs.
Drugs included in this class are:Cefepime (IV)Cefpirome (IV)Cefozopran (IV)

5th Generation Cephalosporins
These 5th generation cephalosporins are active against Methicillin resistant staphylococci.
Agents under this class include:Ceftaroline Fosamil (IV)Ceftobiprole (IV)

FDA has approved Ceftaroline under the trade name Teflaro which was developed by modifying the structure of 4th generation cephalosporin Cefozopran.
Ceftobiprole has powerful antipseudomonal characteristics and appears to be less susceptible to development of resistance and are now on the FDA fast-track.

Currently, ceftaroline and ceftobiprole are on an unnamed subclass of cephalosporins by the Clinical and Laboratory Standards Institute (CLSI) but generally classified under the category of 5th generation cephalosporins.

Resistance to Cephalosporins
Resistance to cephalosporins can be due to following mechanisms:
Poor penetration of drug into bacteriaLack of specific PBPS for a particular
agent Degradation of the drug by β-lactamasesFailure of activation of Autolytic enzymes
in the bacterial cell wall.

Therapeutic Uses
Cephalosporins are widely used antibiotics. Unfortunately, overuse of these agents in situations where drugs with less broad spectrum activity would be more appropriate has led to the emergence of wide array of cephalosporin resistant bacteria.
Cephalosporins are effective as both Prophylactically & Therapeutically.

Chemoprophylaxis
Single dose of Cefazoline (1-2 g IV/IM ≤60 minutes before procedure) just before surgery is preferred prophylaxis for procedures in which skin flora are likely to be pathogenic.

Alternative to Penicillins
Cephalosporins are still useful alternatives to Penicillins for a variety of infections in patients who cannot tolerate penicillins.

Serious Infections
Cephalosporins (Ceftriaxone or Cefixime), alone or in combination with Aminoglycosides are drugs of choice for serious infections (commonly respiratory tract infections) caused by Klebsiella, Enterobacter, Proteus, Providencia, and Haemophilus species.

Treatment of Gonorrhoea
Ceftriaxone (as a single dose 125mg by injection) and Cefixime (400mg oral dose) are drugs of 1st choice for the treatment of all forms of gonorrhoea.

Treatment of Typhoid
Cefoperazone and Ceftriaxone (1-2g BD, IV/IM for 7-10 days) have been used effectively for the treatment of typhoid fever.

Meningitis
Ceftriaxone and Cefotaxime, currently are the drug of choice for empirical treatment of meningitis in non immunocompromised adults and children older than 3 months.
They are proven effective for the treatment of meningitis caused by Haemophilus influenzae, Neisseria meningitidis and gram-negative enteric bacteria.

Treatment of Lyme Disease
Lyme is an inflammatory disease spread by infected Tics bite by Borrelia specie.
Ceftriaxone (2 g once daily
iv for 14–28 days) or
Cefotaxime (2 g iv
every 8 h) is the treatment
of choice for severe forms of Late Lyme Disease.

Adverse Effects
Allergic ReactionsNephrotoxicityDiarrhoeaDisulfiram like ReactionBleeding DisorderSuperinfections

Other β-Lactam Antibiotics
& Cell Wall Destructor

Other β-Lactam Antibiotics
β-Lactamase InhibitorsClavulanic AcidSalbactumTazobactum
Monobactams Aztreonam

Carbapenems Doripenem ImipenemErtapenemMeropenem

β-Lactamase Inhibitors
β-lactamase inhibitors are used in conjunction with a β-Lactam antibiotic to extend its spectrum of activity.
Although β-lactamase inhibitors have little antibiotic activity of their own, they instead inhibit the activity of β-lactamases, (a family of enzymes that break the beta-lactam ring) that allows penicillin-like antibiotics to work, thereby conferring bacterial resistance.

Hence β-lactamase inhibitors are often given in combination with penicillins to tackle the problem of the resistance caused by the presence of β-lactamases from bacterial cells.
An example is Co-Amoxiclav [Augmentin], which is a combination of amoxicillin and clavulanic acid. Salbactum usually combined with Ampicillin (Unasyn) and Tazobactum with Piperacillin (Zosyn).

Monobactams
Monobactams are drugs with a monocyclic β- lactam ring.
They are resistant to β-lactamases and active against gram-negative rods.
They have no activity against gram-positive bacteria or anaerobes.
Penicillin-allergic patients tolerate aztreonam without reaction.

CarbapenemsImipenem has good activity against gram-
negative, gram-positive and anaerobic organisms.
Imipenem is inactivated by dehydropeptidases in renal tubules.
It is administered with an inhibitor of renal dehydropeptidase, cilastatin, for clinical use.
Meropenem and ertapenem are not degraded by renal dehydropeptidase.

IM ertapenem is irritating, so it is formulated with 1% lidocaine.
A carbapenem is used for P. aureginosa resistant to other drugs and mixed aerobic and anaerobic infections.

Cell Wall Destructor
Vancomycin Daptomycin FosfomycinPolymyxinCycloserine

Vancomycin
Vancomycin is active against gram-positive bacteria, particularly staphylococci.
Machanism: Inhibits cell wall synthesis by binding to the D-Ala-D-Ala terminus of peptidoglycan pentapeptide, which as a result inhibits transglycosylase, preventing peptidoglycan elongation and cross-linking.

β-lactamase producing staphylococci and those resistant to nafcillin and methicillin are killed by vancomycin.
Vancomycin is poorly absorbed from the GI tract.

It is used orally only for antibiotic-associated enterocolitis caused by C. difficile.
Metronidazole is preferred as initial therapy and vancomycin is reserved for refractory cases.
Parenteral vancomycin is used in sepsis caused by methicillin-resistant staphylococci.

Vancomycin is irritating to tissue, resulting in phlebitis at the site of injection.
A common reaction is "red man" or "red neck" syndrome.
This infusion-related flushing is caused by release of histamine.
It can be largely prevented by prolonging the infusion period to 1-2 hours or increasing the dosing interval.

Daptomycin
It is similar to vancomycin but is active against vancomycin-resistant enterococci and S. aureus.
It appears to bind to and depolarize the cell membrane, causing potassium efflux and rapid cell death.
It can cause myopathy, so creatine kinase levels should be monitored regularly while individual undergo daptomycin therapy.

Fosfomycin
It inhibits bacterial cell wall biogenesis by inactivating the cytoplasmic enzyme, enolpyruvate transferase.
Fosfomycin is active against both gram-positive and gram-negative organisms.
Fosfomycin is used for treatment of uncomplicated urinary tract infections.

Polymyxins
Polymyxins antibiotic primarily used for resistant Gram-negative infections.
Alters bacterial outer membrane permeability by binding to a lipopolysaccharide layer resulting in disruption of membrane integrity.

Polymyxin B is applied topically to treat infections such as those of the eye, ear, and skin. Polymyxin E, also known as colistin, is used frequently for diarrhea in children.
Because polymyxins also react with the membranes of human cells, they can cause kidney damage and neurotoxicity. The availability of better antibiotics limits the use of polymixins.

Cycloserine
Cycloserine is used only to treat tuberculosis resistant to first-line agents.
Cycloserine causes serious CNS toxicity with headaches, tremors, acute psychosis, and convulsions.

References Katzung Pharmacology, 12th Edition BNF, 61st Edition Christopher Duplessis, and Nancy F. Crum-Cianflone. (2012).
Ceftaroline: A New Cephalosporin with Activity against Methicillin-ResistantStaphylococcus aureus (MRSA). Clin Med Rev. doi: 10.4137/CMRT.S1637
Ito M, Ishigami T. The meaning of the development of flomoxef and clinical experience in Japan. Infection. 1991;19 Suppl 5:S253-7.
http://www.emedexpert.com/