Biology 638 – Biochemistry II Exam-1 - University of...
Transcript of Biology 638 – Biochemistry II Exam-1 - University of...

Biol 638, Exam-1 (Code-1)
Biology 638 – Biochemistry II Exam-1
Using the following values, answer questions 1 - 3. ATP + H2O ↔ ADP + Pi ΔG°’ = -30 kJ/mol
Creatine-phosphate + H2O ↔ Creatine + Pi ΔG°’ = -12 kJ/mol ½O2 + 2H+ + 2e- ↔ H2O ΔE°’ = 0.80 V FAD + 2H+ + 2e- ↔ FADH2 ΔE°’ = -0.2 V R = 10.0 J·mol-1· K-1 F = 100.0 kJ·V-1 T = 300 K ln(x) = 2log(x) In the calculation questions, select the closest one.
1. Creatine kinase catalyzes the following reaction. ATP + creatine ↔ ADP + creatine-phosphate The ΔG of the above reaction is _______ kJ/mol under the biological standard conditions. a. -40 b. -20 c. -10 d. 20 e. 40 2. pH values of the inside (matrix) and outside (intermembrane space) of mitochondrion are 9 and 5,
respectively. The electropotential between the inside and outside is 0.30 V (inside is negative). This electrochemical potential is _______ kJ/mol. a. -10 b. -20 c. -30 d. -40 e. -50
3. O2 is reduced by FADH2 to H2O in a mitochondrion by the following reaction: ½O2 + FADH2 ↔ H2O + FAD
If the all oxidation energy produced in the above reaction is utilized to synthesize ATP from ADP + Pi, how many ATP would be synthesized from five FADH2 oxidation under standard biological conditions?
a. 5 b. 15 c. 20 d. 25 e. 30
1

Biol 638, Exam-1 (Code-1)
4. A person’s urine contains abnormal level of phenylpyruvate. This person might have ____ degradation problem.
a. pyruvate b. Gly c. Phe d. Tyr e. heme
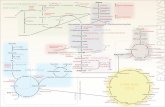
The figure below is the glycolysis pathway. Using the figure, answer the following three questions.
E5
E9 E8 E7
E6
Pyruvate M8M9
E4
M6
M5E3E2E1
M4M3M2Glucose
M7M10E10
5. Which step(s) produce ATP? a. E3, E10 b. E7, E10 c. E6, E9 d. E3, E8 6. ______ step is the major regulation site of glycolysis. a. E3 b. E4 c. E5 d. E1 7. What is M7? a. Dihydroxyacetone phosphate b. 1,3-Bisphosphoglycerate c. 2-Phosphoglycerate d. 3-Phosphoglycerate 8. Glycolysis influences oxygen transport. Select the correct statement. a. Fast glycolysis decreases the HbO2 concentration.
b. Slow glycolysis increases the HbO2 concentration. c. Hexokinase deficient results in high HbO2 concentration. d. Pyruvate kinase deficient results in low HbO2 concentration.
e. All of the above. 9. 2PG is converted to PEP because _____.
a. conversion of 2PG to PEP is a large exergonic process to pull the previous reaction 3PG to 2PG b. PEP has a higher energy molecule than 2PG c. conversion of 2PG to pyruvate requires many steps d. conversion of PEP to pyruvate is a large exergonic process to produce an ATP e. None of the above.
2

Biol 638, Exam-1 (Code-1)
10. It is well-known that nitroglycerin works on angina pectoris because _____. a. it increases the IP3 level in heart smooth muscle b. it increases the cAMP level in heart smooth muscle c. it produces NO in heart smooth muscle d. it elevates the Ca2+ concentration in heart smooth muscle e. None of the above
11. ____ has (have) kinase activity. a. Insulin receptor b. Steroid receptors c. α-Adrenoreceptors (phosphoinositide pathway receptors) d. β-Adrenoreceptors e. None of the above. 12. Caffeine inhibits ______. a. GTP hydrolysis on Gsα b. cAMP hydrolysis to AMP c. Giα from exchanging its bound GDP for GTP d. GTP hydrolysis on Giα e. None of the above. 13. Adenylate cyclase is activated by _______. a. Ca2+ b. Gqα⋅GTP c. cAMP d. Gsα⋅GTP For question 14 – 25, select that statement that contains the most significant mistake(s). If none of (a, b, c, d) does not contain significant mistake(s) (i.e. all statements are correct), then select “e. None of the above.” 14. The followings are some characteristics of ATP molecule. a. Hydrolysis of α-phosphate of ATP produces more energetic than that of γ-phosphate. b. Triphosphate moiety of ATP is relatively unstable because its solvation energy is small.
c. Triphosphate moiety of ATP is relatively unstable because its resonance stabilization is small. d. ATP occupies the middle rank of phosphoryl-transfer energy production. e. None of the above. 15. The followings are some characteristics seen in a metabolism introduction.
a. Phosphoguanidines have a high energy bond. b. Complete hydrolysis of ATP (ATP → AMP) produces more than 60 kJ/mol under the standard
biological condition. c. X-ray irradiation gives damages on bacterial DNA and produces various auxotropic mutant
bacteria. d. The nitrogen atoms of heme in mammals are supplied from Gly. e. None of the above.
3

Biol 638, Exam-1 (Code-1)
16. The followings are catalytic functions of enzymes. a. A-phosphorylase catalyzes: A-O-B + Pi → A-Pi + HO-B b. A-kinase catalyzes: A + ATP → A-Pi + ADP c. G6P → F6P is catalyzed by a isomerase, while G6P → G1P is by a mustase. d. Alcohol to aldehyde conversion is catalyzed by a dehydrogenase if NAD+ is involved in the
reaction. e. None of the above
17. The followings are some characteristics of enzymes in glycolysis.
a. Glyceraldehyde-3-phosphate dehydrodenase is inhibited by iodoacetate because it has an essential Cys in the active site.
b. Phosphoglycerate mutase has a phosphorirated Ser in the active site. c. Phosphoglucose isomerase has two essential amino acid residues, Lys and Glu, in the active site. d. Aldolase has two essential amino acid residues, neutral Lys and negatively charged Tyr, in the
active site. e. A large conformational change has been observed in hexokinase during the catalytic reaction.
18. The followings are some characteristics in glycolysis and related enzymes. a. The catalytic rate of triose phosphate isomerase (TIM) is very fast, so that the GAP concentration
is properly regulated. b. Alcohol dehydrogenase is a zinc containing protein. c. Pyruvate decarboxylase requires a cofactor NAD+. d. The equilibrium constant, K = [GAP]/[DHAP] is very low because GAP inhibits aldolase. e. None of the above.
19. The followings are some characteristics in glycolysis.
a. Conversion of pyruvate to lactate regenerates NAD+ to continue glycolysis. b. Although the glyceraldehyde 3-phosphate dehydrogenase reaction is an endergonic, it occurs
because the following phosphoglycerate kinase reaction is a large exergonic. c. If G6P is not converted to F6P, the aldolase cleavage would give the C2 and C4 fragments. d. Glycolysis occurs in cytosol. e. None of the above.
20. The followings are some descriptions of hexoses.
a. Mannose is phosphorylated by hexokinase. b. Glucose is phosphorylated by hexokinase. c. Galactose is phosphorylated by hexokinase. d. Fructose in muscle is phosphorylated by hexokinase. e. None of the above.
4

Biol 638, Exam-1 (Code-1)
5
21. The followings are some characteristics in glycolysis. a. The active site of PFK has a Cys because addition of iodoacetate in glycolysis causes
accumulation of fructose-1,6-bisphosphate (FBP). b. If enolase is moderately inhibited, the deoxy-Hb concentration is increased in blood.
c. If TIM is moderately inhibited in glycolysis, the FBP concentration is increased. d. Conversion of one glucose to two pyruvates in glycolysis produces two ATP’s and two NADH’s. e. None of the above. 22. Glycolysis is largely regulated by the following enzyme actions.
a. Large conformation change of hexokinase b. Allosteric activation/inactivation of phosphofructokinase
c. AMP concentration by adenylate kinase d. Hydrolysis by fructose-1,6-bisphosphatase e. None of the above
23. If adenylate kinase is inhibited in glycolysis, ________. a. the concentration of AMP will be decreased b. the activity of fructose-1,6-bisphosphatase will be decreased c. the activity of phosphofructokinase will be decreased d. the concentration of ATP will be decreased e. None of the above. 24. In α-adrenoreceptor (phosphoinositide pathway receptor) signal transduction pathway, ____ plays an
important role. a. phospholipase C
b. calmodulin c. Gqα
d. protein kinase A e. None of the above
25. The signal of epinephrine is naturally reduced by _____.
a. hydrolysis of cAMP b. separation of protein kinase A (R2C2 → R2 + C2)
c. hydrolysis of GTP on Gsα d. replacement of GDP with GTP on Giα e. None of the above.