Abstract - The National Laboratory Association | National ... · Web viewThis this study present...
Transcript of Abstract - The National Laboratory Association | National ... · Web viewThis this study present...

Determination of Endocrine Disrupting Compounds such as Hormones in Environmental Water Samples by HPLC-MS
Speaker(s)/Author: S. MnguniCo-Author(s): C. Schoeman
Rand Water Analytical ServicesPO Box 3526, Vereeniging,1930, South Africa
e-mail:[email protected]: 016 430 8408 Fax: 016 455 2055
Abstract
This paper present the development of a method for determination of four hormones in water samples, due to their toxicity effects hormones such as Estrone (E1), 17β-Estradiol (E2), 17α -Estradiol (E3) and 17α-Ethinylestradiol (EE2), possess endocrine disruption potential even at trace concentration levels.
Hormones in water samples have been classified as “emerging pollutants”; these compounds enter water streams through waste water treatment plants (WWTP) and they can be found in both surface and ground water. A significant feminization could be observed at a concentration of approximately 1 ng/L reflecting the strong endocrine disrupting potential of these compounds. As a result of these low concentrations a suitable analytical methodology is essential for their determination.
In the past residues of estrogens in aqueous and solid environmental samples have been commonly analysed by gas chromatography (GC) equipped with mass spectrometer, however the necessary derivatisation steps are not only time consuming and laborious but also tedious.
This study utilises a sensitive analytical methodology for the analysis of these hormones in source and processed water samples at ultra-trace concentration levels. Analytes were extracted by traditional solid phase extraction (SPE) and consequently injected into Liquid chromatography coupled to mass spectrometer (HPLC-MS) for analyte determinations.
The limits of quantification were 0.20 ng/L for all analytes, recoveries were 95.6, 93.8, 97.6 and 100.1 % for 17 α Ethinylestradiol, 17 α-Estradiol, 17β- Estradiol and Estrone respectively.
Treated potable water samples do not contain detectable levels of hormones, this is due to the stringent Randwater’s treatment process, and endocrine disruptors are removed during water purification.

1. Introduction
This this study present the development and validation of a method for determination of four hormones in water samples, due to their toxicity effects hormones such as Estrone (E1), 17- α -Estradiol (E2(α)), 17-β -Estradiol (E2(β)) and 17α-Ethinylestradiol (EE2), possess endocrine disruption potential even at trace concentration levels.
Herein we developed and validated a new method for simultaneous determination of hormones is environmental water samples using a highly sophisticated high performance liquid chromatograph coupled to triple quadrapole mass spectrometer.
The objective of this study is to validate the method and to determine the fundamental figures of merit such as; - (1) Limit of detection, LOD, (2) Limit of quantification, LOQ, (3) Accuracy, (4) Precision and (5) Uncertainty of measurements.
This study is intended to confirm the fitness for purpose status of the hormones method.
2. Literature review
The occurrence of human pharmaceuticals and endocrine disrupting compounds (EDCs) in wastewaters and in surface waters, Richardson et al, (2007) has been demonstrated and widely evaluated in the last decade. These compounds together with their metabolites enter the environment primarily through domestic use to sewage treatment plants. If these compounds are not quantitatively eliminated, they can be then discharged to rivers and lakes which may be used as raw sources for drinking water production. Therefore the presence of such organic contaminants might have a negative impact on the quality of drinking water together with unknown toxicological effects through chronic exposure [1].
A significant feminization could be observed at a concentration of approximately 1 ng/L reflecting the strong endocrine disrupting potential of these compounds. As a result of these low concentrations a suitable analytical methodology is essential for their determination [1,6].
In the past residues of estrogens in aqueous and solid environmental samples have been commonly analysed by Gas Chromatography (GC) equipped with Mass Spectrometer (MS), however the necessary derivatisation steps are not only time consuming and laborious but also tedious [1,7].
In view of this scene, the incidence of pharmaceuticals and hormones in raw waters used for drinking water production and their elimination through treatment must be considered an issue in terms of health safety for water production. However, compared to wastewater treatment plants, much less is known about the behavior of these drugs in drinking water treatment plants or their presence in tap waters. The lack of systematic monitoring programs and also the fact that they are present at fluctuating concentrations near analytical method detection limits (some of these compounds usually occur in the low ng/L range or below) could be some reasons to explain the relative little knowledge of the occurrence of these compounds, their metabolites and their transformation products through drinking water treatment [1].

Recently, Jones et al. (2005) and Mompelat et al. (2009) reviewed the occurrence and fate of pharmaceuticals and transformation products in drinking waters.
To date, several EDCs have been found in European and USA’ tap waters at concentrations ranging from ng/L level to low mg/L [1].
The high performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) is able to simultaneously analyse multiple target analytes within on sample run. It is shown over the past ten years that HPLC–MS/MS is a reliable standard technique in clinical application fields like therapeutic drug monitoring (TDM), newborn screening, and toxicological diagnostics. Therefore, HPLC–MS/MS has the potential to become a strong competitor for widely used immunoassays for steroid analysis, especially when more than one steroid hormone has to be analysed with high selectivity, sensitivity, precision and accuracy [2].
Main disadvantages of immunoassays or complex matrices, particularly relevant for steroid hormone analysis [8] are as follows:
Measurement of one analyte at a time in one processed sample. Lack of antibody specificity due to cross reactivities with structurally similar
metabolites (poor accuracy) especially at low concentration ranges, which can result in diagnostic errors e.g. in pediatric range of application.
Limitation in sensitivity (e.g. testosterone in both premenopausal and postmenopausal women).
Matrix interferences, which cannot be corrected by internal standards. - Limited dynamic range.
High variabilities among different immunoassay kits (resulting in significant variations in reference intervals) and among different laboratories employing the same kit due to poor and diverse validation and standardisation data.
However, HPLC–MS/MS needs comprehensive standardisation to become a successfully established technology in clinical routine laboratories for steroid hormone analysis. Standardisation is needed to minimize analytical intra- and inter-laboratory variabilities (imprecision and inaccuracy) and therefore, to improve the quality of the quantitative results [6-8].
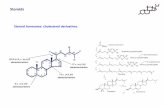
Figure 1. Structure of Hormones
3. Experimental

3.1. Reagents and samples
Estrone (E1, > 99.0 %), 17β-Estradiol (E2, > 99.0 %), 17α -Estradiol (E3, > 99.0 %) and 17α-Ethinylestradiol (EE2, > 99.0 %) were purchased from Dr Ehrestorfer GmbH, Germany. Methanol (99.9 %), GC& HPLC grade was purchased from Merck KGaA, SA. Acetonitrile (99.9 %) and deionised water (Honeywell), HPLC grade were purchased from Burdick & Jackson. Deionised water for blank determination was purified in a Milli Q system Millipore.
Standard stock solutions were prepared by dissolving 10 mg of each hormone compound into a 10 ml volumetric flask using a 50 % methanol solution. A mixed standard containing concentration of 0.01 ng/µl was prepared following further dilutions. A multi calibration solution containing target compounds at concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 ng/L was prepared in I L of deionised water prior extraction.
Samples were collected from catchment, raw and potable water sampling points in February 2013. A quantity of 1 L of each sample was extracted and analysed in the same manner as calibration standards.
3.2. Instrumentation
A Shimadzu HPLC System equipped with Auto Sampler and external column oven was used for separation. A volume of 100 µL of each sample was introduced into a HPLC and separated on a Phenomenex Kinetex C18 (100 x 4.6 mm; 2.6 μm) column (including guard column) at 40 ºC. The gradient elution between acetonitrile (containing 5 % water) and water (containing 5 % acetonitrile) was performed at 0.35 mL/min. The gradient elution started with 5% Acetonitrile and increased to 85 % over 9 minutes followed by further 3 minutes at 85 % Acetonitrile.
An AB Sciex Q Trap, MS-MS system was used. The mass spectrometer was optimized in negative (M+H-) MRM (Multiple Reaction Monitoring modes), the product ions, that yielded high sensitivity were used for quantitative analysis. Their corresponding declustering potentials (DP), collision energies (CE) and exit potentials (EP) were recoded accordingly.The identification of analyte peaks were further confirmed by using Retention Times (RTs).
3.3. Procedure (Solid Phase Extraction – SPE)
SPE (Agela PEP, 200 mg/6ml) cartridges were conditioned with 3 aliquots of methanol in an assembled SPE manifold. An amount of 10 ml of reagent water was then added to each cartridge followed by elution of 1 litre samples at a flow rate of 10mL/min under small vacuum.
Cartridges containing extracted analytes were further dried under full vacuum for about 10 to 15 minutes followed by the slow elution of analyte into collection tubes. A total volume of 7.5 mL of methanol was used to elute analytes from the SPE cartridge.
The extract was then concentrated to dryness with nitrogen before adjusting to a 250 µl volume with a 50% methanol solution. An aliquot was injected into a HPLC interfaced to an MS.4. Results and Discussion

Aliquots were injected into a HPLC interfaced to an MS. Analytes were identified by comparing the mass spectra and retention times to reference spectra for calibration standards acquired under identical HPLC-MS conditions. The concentration of each analyte was determined using the external standardisation technique. Post column introduction of 0.4% NH3 at a flow rate of 0.05 ml/min was performed using a Thermo pump system.
Four compounds identified were chosen as target analytes for this study due to the availability of standards. They belong to the one class of estrogens and are shown in Figure 1 below. Estriol was not investigated in this study due to unavailability of a standard.
The advantage in this study is the ability of the SPE method to extract simultaneously all target compounds and the ability of LC-MS to detect lowest levels of these compounds along the Vaal river catchment area.
In order to extract hormones in water, physical properties such as pKa and Log Kow are needed to be taken into account since they govern prevalence of ionic of neutral nature of compounds as function of the pH [1]. See table 1 below.
Table 1. Physico-chemical properties and parameters of hormones
Analyte pKa Log K ow r2Linearity
(ng/L)LOD (LOQ)
ng/LE1 10.7 3.7 0.998 0-1 0.07 (0.24)
E2(α) 10.7 4.1 0.997 0-1 0.09 (0.30)E2(β) 10.7 4.1 0.996 0-1 0.10 (0.32)EE2 10.2 4.5 0.998 0-1 0.07 (0.24)
Properties of hormones were obtained from (Carpinteiro et al, 2004 and Lewis and Archer, 1979)
Each pure hormone component was subjected onto a high performance liquid chromatography coupled to mass spectrometer, for qualitative purposes and each component were identified by its retention time and by comparing the mass to reference spectra for calibration standards acquired under identical HPLC-MS conditions. Two isomers of estradiol eluted at the sample retention times.
The mixture of hormones was analysed and only three peaks were observed due to co-elution of 17β-Estradiol (β-E2) and 17α -Estradiol (α-E2) because of their chemical properties hence conditions were changed to achieve the separation (Figure 2)

Figure 2. Typical Chromatogram of a mixture of hormones in 50 % methanol in their elution order E2 (α), E2 (β), EE2 and E1.
A quality control sample of 0.50 ng/L was analysed over the period of three non-consecutive days, calibration standards were prepared daily prior analysis.
Based on the evidence in Table 2, the average precision for all compounds falls within 15%, this value is acceptable given the ultra-trace nature of these analytes and complexity of the method. The accuracy is within ±10% semi-range for most hormones which is also acceptance for the same reason.
Table 2. Method performance parameters obtained from statistical evaluation.
Analyte
Precision (% RSD)
Accuracy(%)
Uncertainty (ng/L), +/-
Catchment Sample (ng/L)
Raw water (ng/L)
Potable water (ng/L)
E1 9.3 100.9 0.15 ND 0.24 NDE2(α) 9.6 93.8 0.14 ND ND NDE2(β) 11.4 97.6 0.15 ND ND NDEE2 10.7 95.6 0.14 ND ND ND
ND – is applicable to analytes not detected.
There were no detectable levels of hormones in samples analysed with one exception of Raw water samples where 0.24 ng/L of Estrone was detected. Treated potable water sample did not contain detectable levels of hormones, this is due to the stringent Randwater’s treatment process, and endocrine disruptors are removed during purification. Regarding water analysis, earlier studies have shown that these compounds are only partially eliminated during conventional (coagulation, sand filtration) wastewater treatment [1, 2].
This method is applicable and linear up to 1.00 ng/L concentration levels, samples that fall beyond the linear calibration range are diluted accordingly.

5. Conclusion
Based on statistical analysis the high accuracy, good precision, limits of detection, limits of quantification and overall good performance in terms of consistency in results obtained by SPE and HPLC interfaced to a Mass Spectrometer, Consequently the evidence demonstrates that the developed method is fit for purpose.
Acknowledgements
This study is sponsored by Rand Water as their strategic initiative towards monitoring of endocrine disrupting compounds in environmental samples.
6. References
1. Maria Huert-Fontel, Maria Teresa Galceran, Frances Ventura, Occurrence and removal of pharmaceuticals and hormones through drinking water treatment, 2010
2. Theres Koal, et al, Standardized LC–MS/MS based steroid hormone profile-analysis., 2011
3. J.-Y. Pailler, et al, Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in Luxembourg, , 2009
4. Chung Chow Chan, Y.C.Lee, Herman Lam, Xue-Ming Zhang, Analytical Method Validation and Instrument Performance Verification, , A John Wiley& Sons, Inc., Publication, first edition, 2004.
5. Ali Khan, et al, The development and application of a system for simultaneously determining anti-infectives and nasal decongestants using on-line solid-phase extraction and liquid chromatography–tandem mass spectrometry, 2011
6. Yoshihiro Izumi, et al, Development of a method for comprehensive and quantitative analysis of plant hormones by highly sensitive nanoflow liquid chromatography–electrospray ionization-ion trap mass spectrometry, 2009.
7. Maria Huert-Fontel, Maria Teresa Galceranb, Francesc Venturaa, Fast liquid chromatography–quadrupole-linear ion trap mass spectrometry for the analysis of pharmaceuticals and hormones in water resources, 2009.
8. Sabine Mu¨llera , et al, D-04318 Leipzig, Germany bUFZ Centre for Environmental Research Leipzig-Halle, Semi-automated hollow-fibre membrane extraction, a novel enrichment technique for the determination of biologically active compounds in water samples, Permoserstrasse 15, D-04318 Leipzig, Germany, 2002