A New β 0 Frameshift Mutation, HBB : c.44delT (p.Leu14ArgfsX5), Identified in an Argentinean Family...
Transcript of A New β 0 Frameshift Mutation, HBB : c.44delT (p.Leu14ArgfsX5), Identified in an Argentinean Family...

http://informahealthcare.com/hemISSN: 0363-0269 (print), 1532-432X (electronic)
Hemoglobin, Early Online: 1–3! 2014 Informa Healthcare USA, Inc. DOI: 10.3109/03630269.2014.964361
SHORT COMMUNICATION
A New b0 Frameshift Mutation, HBB: c.44delT (p.Leu14ArgfsX5),Identified in an Argentinean Family Associated with SecondaryGenetic Modifiers of b-Thalassemia
Carolina Pepe, Silvia Eandi Eberle, Alejandro Chaves, Berenice Milanesio, Fernando M. Aguirre, Vanesa AvalosGomez, Lilian Diaz, Adrian P. Mansini, Diego A. Fernandez, Gabriela Sciuccati, Andrea Candas, Carolina Cervio,Mariana Bonduel, and Aurora Feliu-Torres
Servicio de Hematologıa-Oncologıa, Hospital de Pediatrıa ‘‘Prof. Dr. Juan P. Garrahan’’, Buenos Aires, Argentina
Abstract
b-Thalassemia intermedia (b-TI) patients present with a wide spectrum of phenotypesdepending on the presence of primary, secondary, and tertiary genetic modifiers whichmodulate, by different mechanisms, the degree of imbalance between a and b chains. Here wedescribe a new b0 frameshift mutation, HBB: c.44delT (p.Leu14ArgfsX5), identified in fourmembers of a family, associated with secondary genetic modifiers in three of them. Thedifferent genotype present in this family was suspected after hematological analysis andthorough observation of blood smears highlighting their importance in the identification ofb-TI patients among members of the same family.
Keywords
b-Thalassemia intermedia (b-TI), geneticmodifiers, new mutation
History
Received 8 April 2014Accepted 2 May 2014Published online 30 September 2014
The clinical manifestations of b-thalassemia (b-thal) are
extremely variable in severity, ranging from the transfusion-
dependent b-thal major (b-TM) to the asymptomatic carrier
state. Somewhere in between these two different entities is
b-thal intermedia (b-TI), with a wide spectrum of phenotypes
and transfusion requirement (1). The phenotypic diversity of
b-TI depends on the existence of primary, secondary, and
tertiary genetic modifiers which modulate, by different
mechanisms, the degree of imbalance between a and bchains, finally determining the patient’s clinical and hemato-
logical manifestations (2).
The primary genetic modifiers are the mutations present on
the HBB gene, for which more than 800 genetic variants have
been previously described (3). These mutations are very
heterogeneous, with some of them associated with no b chain
production from the affected b allele (b0-thal) and others
allowing b chain synthesis at a reduced rate (b+-thal) (4). The
production of a and g chains is modulated by secondary
modifiers, such as coinheritance of a-thalassemia (a-thal),
presence of triplicate/quadruplicate a genes, and increased gchain production associated with more elevated levels of
Hb F. Hb F levels are regulated by three major loci: HBG2: c.-
211 C4T (Gg-158 C>T or XmnI polymorphism) on 11p15.4
inside the b-globin gene cluster, HBS1L-MYB intergenic
region on 6q23.3 and BCL11A on 2p16.1 (5).
A novel HBB frameshift mutation was identified in an
Argentinean family associated with secondary genetic modi-
fiers whose presence may ameliorate or worsen the b-thal
phenotype. The proband, a 19-year-old female of Spanish
ancestry, was born after an uncomplicated full-term preg-
nancy and delivery. She had suffered from anemia since
childhood receiving iron therapy on multiple occasions. On
admission to our institution, her physical examination was
unremarkable except for pallor. The proband’s family (her son
and two brothers) were also examined. Hematological data
were obtained with a Sysmex XS800i (Sysmex Corporation,
Kobe, Japan). Hemoglobin (Hb) electrophoresis was carried
out with a semiautomatic agarose-gel system at an alkaline
pH (Sebia, Lisses, Evry, France). Hb A2 was measured by
anion exchange chromatography (6) and Hb F according to
the method described by Betke et al. (7).
The HBB gene was amplified and sequenced from DNA
isolated from peripheral blood leukocytes using primers
previously described by Roldan et al. (8). Exonic and intronic
flanking regions were directly sequenced with the Sanger
method and an automated capillary sequencer ABI PRISM
3130 (Applied Biosystems, Buenos Aires, Argentina). The
most frequent deletions on the a-globin cluster [�a3.7
(rightward) and �a4.2 (leftward)] were analyzed with gap-
polymerase chain reaction (gap-PCR) using primers and a
strategy described by Chong et al. (9), with some modifica-
tions. Briefly, 100 ng of genomic DNA was amplified using
200 mM dNTPs, primers, 1� PCR buffer, 1�QSolution
Address correspondence to Dr. Aurora Feliu-Torres, Servicio deHematologıa-Oncologıa, Hospital de Pediatrıa ‘‘Prof. Dr. Juan P.Garrahan,’’ Combate de los Pozos 1881, (C1245AAM) Buenos Aires,Argentina. Tel: +54-11-4308-4300, Ext: 1301-1597. Fax: +54-11-4308-5325. E-mail: [email protected]
Hem
oglo
bin
Dow
nloa
ded
from
info
rmah
ealth
care
.com
by
The
Uni
vers
ity o
f M
anch
este
r on
10/
13/1
4Fo
r pe
rson
al u
se o
nly.

(Qiagen, Buenos Aires, Argentina), and 1.25 U of Hot Star
Taq DNA polymerase (Qiagen) in 25 mL of reaction volume.
After amplification, 10 mL of product was analyzed by
electrophoresis in 1.0% agarose gel stained with ethidium
bromide. The presence of the anti-3.7 a-globin gene
triplication was analyzed using PCR as previously described
(10). The number of a genes present on the anti-3.7 allele, the
excess of a-globin genes generated by other mechanisms, and
the presence of different deletions on the a-globin gene
cluster were studied by multiplex ligation-dependent probe
amplification (MLPA) using the commercial kit Salsa MLPA
P140B HBA (MRC-Holland, Amsterdam, The Netherlands)
following the manufacturer’s instructions. The genetic variantGg-158 was analyzed by PCR-RFLP (restriction fragment
length polymorphism) using the XmnI enzyme (11).
Hematological and molecular findings are shown in
Table 1. Hematological results for all individuals suggested
a diagnosis of b-thal trait. In addition to anisocytosis,
microcytosis, and hypochromia, individuals with a more
severe phenotype (the proband, her son, and one of her
brothers) have moderate-to-severe poikilocytosis with promi-
nent basophilic stippling. Hb A2 percentage was increased in
all individuals.
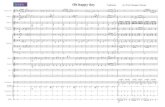
A new mutation, HBB: c.44delT (p.Leu14ArgfsX5) was
identified in all members of the family analyzed. It results
from a single nucleotide deletion at codon 14. The deletion
determines a frameshift and the generation of a premature
termination codon (codon 18) (Figure 1). The resultant
mRNA could be targeted to nonsense-mediated decay or
could be translated to a very short peptide that would be
completely hydrolyzed by the proteolytic system of red blood
cells (RBCs), resulting in no protein being synthesized from
the affected allele (12,13).
An increased level of Hb F was observed in the proband’s
son, who is homozygous for the Gg-158 T allele. The other
family members with only one T allele have normal or slightly
increased Hb F levels. It has been described that the effect of
the �158 determinant is not consistent, as normal levels of
Hb F have been found both in b-thal heterozygotes and in non
thalassemic individuals. Nevertheless, in conditions of
erythropoietic stress, the Gg-158 genetic marker could lead
to increased Hb F production, playing a role in the
amelioration of the phenotype (14).
The analysis of the a-globin gene cluster revealed the
absence of deletions inside the cluster but the presence of an
extra a-globin gene in the proband, her son, and one of her
brothers. This extra a-globin gene increases the a/b-globin
chain imbalance impairing the phenotype of these three
patients.
In conclusion, we describe a new b0 mutation identified in
a heterozygous state in an Argentinean family. In the proband,
Table 1. Hematological and molecular studies (RBC: red blood cell; Hb: hemoglobin; MCV: mean corpuscular volume;MCH: mean corpuscular Hb).
Parameters Proband Son Brother Brother
Sex-Age (years) F-19 M-2 M-15 M-14RBC (1012/L) 4.95 5.49 5.99 5.64Hb (g/dL) 9.0 10.3 11.2 11.4MCV (fL) 64.0 54.5 55.1 62.5MCH (pg) 19.2 18.8 18.4 20.2Hb A2 (%) 5.0 4.4 5.7 6.0Hb F (%) 0.25 14.4 1.70 2.50Blood film:� anisocytosis [+ +] [+ +] [+ +] [+ +]� macrocytosis [+] – [+] [+]� microcytosis [+ + +] [+ +] [+ + +] [+ + +]� hypochromia [+ + +] [+ +] [+ + +] [+ + +]� basophilic stippling [+ +] [+ +] [+ +] [+]� elliptocytes [+ +] [+] [+ +] [+ +]� ovalocytes [+ +] [+] [+ +] [+ +]
Serum ferritin (ng/mL) 49.8 55.7 88.4 72.9b Genotype HBB: c.44delT/bA HBB: c.44delT/bA HBB: c.44delT/bA HBB: c.44delT/bA
a Genotype aaa/aa aaa/aa aaa/aa aa/aaGg Genotype HBG2: c.-211 C/T
(Gg -158C/T)HBG2: c.-211T/T
(Gg -158T/T)HBG2: c.-211C/T
(Gg -158C/T)HBG2: c.-211C/T
(Gg -158C/T)
Figure 1. DNA sequencing of the HBB gene in the proband, her son, andher brothers shows the frameshift generated by a T deletion at codon 14,resulting in the premature stop codon at position 18 of amino acidsequence.
2 C. Pepe et al. Hemoglobin, Early Online: 1–3
Hem
oglo
bin
Dow
nloa
ded
from
info
rmah
ealth
care
.com
by
The
Uni
vers
ity o
f M
anch
este
r on
10/
13/1
4Fo
r pe
rson
al u
se o
nly.

one of her brothers, and her son, the b0 mutation was
coinherited with the anti-3.7 a-globin gene triplication, while
in the other brother it was coinherited with a normal set of
a-globin genes. The analysis of the hematological character-
istics denotes a milder disorder of RBCs in the brother with a
normal set of a-globin genes, highlighting the importance of
thorough observation of blood smears to detect phenotypic
differences among members of the same family in the
identification of patients with b-TI.
Declaration of interest
The authors report no conflicts of interest. The authors alone
are responsible for the content and writing of this article.
References
1. Galanello R, Sanna S, Perseu L, et al. Amelioration of Sardinian b0
thalassemia by genetic modifiers. Blood. 2009;114(18):3935–3937.2. Galanello R. Recent advances in the molecular understanding
of non-transfusion-dependent thalassemia. Blood Rev. 2012;26(Suppl 1):S7–S11.
3. Patrinos GP, Giardine B, Riemer C, et al. Improvements in theHbVar database of human hemoglobin variants and thalassemiamutations for population and sequence variation studies. NucleicAcids Res. 2004;32(Database issue):D537–D541 (http://globin.cse.psu.edu).
4. Bain BJ. Haemoglobinopathy Diagnosis (Second Edition). Oxford,UK: Blackwell Publishing, 2006.
5. Danjou F, Anni F, Perseu L, Satta S, et al. Genetic modifiers ofb-thalassemia and clinical severity as assessed by age at firsttransfusion. Haematologica. 2012;97(7):989–993.
6. International Committee for Standardization in Hematology.Recommendations for select methods for quantitative estimationof Hb A2 and for Hb A2 reference preparation. Br J Hematol 1978;38(4):573–578.
7. Betke K, Marti HR, Schlicht I. Estimation of small percent-ages of foetal haemoglobin. Nature. 1959;184(Suppl 24):1877–1878.
8. Roldan A, Gutierrez M, Cygler A, et al. Molecular characterizationof b-thalassemia genes in an Argentine population. Am J Hematol.1997;54(3):179–182.
9. Chong SS, Boehm CD, Cutting GR, Higgs DR. Simplifiedmultiplex-PCR diagnosis of common Southeast Asian deletionaldeterminants of a-thalassemia. Clin Chem. 2000;46(10):1692–1695.
10. Wang W, Ma ES, Chan AY, et al. Single-tube multiplex-PCRscreen for anti-3.7 and anti-4.2 a-globin gene triplications. ClinChem. 2003;49(10):1679–1682.
11. Lanclos KD, Oner C, Dimovski AJ, et al. Sequence variations in the50 flanking and IVS-II regions of the Gg- and Ag-globin genes of bS
chromosomes with five different haplotypes. Blood. 1991;77(11):2488–2496.
12. Peixeiro I, Silva AL, Romao L. Control of human b-globin mRNAstability and its impact on b-thalassemia phenotype. Haematolo-gica. 2011;96(6):905–913.
13. Romao L, Inacio A, Santos S, et al. Nonsense mutations in thehuman b-globin gene lead to unexpected levels of cytoplasmicmRNA accumulation. Blood. 2000;96(8):2895–2901.
14. Cao A, Moi P, Galanello R. Recent advances in b-thalassemias.Pediatr Rep. 2011;3(2):65–78.
DOI: 10.3109/03630269.2014.964361 A New �0 Mutation with Secondary Genetic Modifiers 3
Hem
oglo
bin
Dow
nloa
ded
from
info
rmah
ealth
care
.com
by
The
Uni
vers
ity o
f M
anch
este
r on
10/
13/1
4Fo
r pe
rson
al u
se o
nly.