3.4 Phospho- and Glycolipids - 123foodscience-food … monosaccharides by orcinol-FeCl 3, choline...
Click here to load reader
Transcript of 3.4 Phospho- and Glycolipids - 123foodscience-food … monosaccharides by orcinol-FeCl 3, choline...

178 3 Lipids
3.3.2.2 Physical Properties
MG and DG crystallize in different forms(polymorphism; cf. 3.3.1.2). The melting pointof an ester of a given acid increases for the series1,2-DG < TG < 2-MG < 1,3-DG < 1-MG:
Melting Point (◦C)β-form
Tripalmitin 65.51,3-Dipalmitin 72.51,2-Dipalmitin 64.01-Palmitin 77.02-Palmitin 68.5
MG and DG are surface-active agents. Theirproperties can be further modified by esterifi-cation with acetic, lactic, fumaric, tartaric orcitric acids. These esters play a significant role asemulsifiers in food processing (cf. 8.15.3.1).
3.4 Phospho- and Glycolipids
3.4.1 Classes
Phospho- and glycolipids, together with proteins,are the building blocks of biological membranes.Hence, they invariably occur in all foods ofanimal and plant origin. Examples are compiledin Table 3.17. As surface-active compounds,phospho- and glycolipids contain hydrophobicmoieties (acyl residue, N-acyl sphingosine) andhydrophilic portions (phosphoric acid, carbohy-drate). Therefore, they are capable of formingorderly structures (micelles or planar layers) inaqueous media; the bilayer structures are foundin all biological membranes. Examples for thecomposition of membrane lipids are listed inTable 3.18.
3.4.1.1 Phosphatidyl Derivatives
The following phosphoglycerides are derivedfrom phosphatidic acid. Phosphatidyl cholineor lecithin (phosphate group esterified with the
Fig. 3.10. The effect of climate (temperature) on thefatty acid composition of triacylglycerols
Table 3.17. Composition of lipids of various foodsa
Milk Soya Wheat AppleTotal lipids 3.6 23.0 1.5 0.088
Triacylglycerols 94 88 41 5Mono-, and diacylglycerols 1.5 1Sterols < 1 1 15Sterol esters 1 2Phospholipids 1.5 10 20 47Glycolipids 1.5 29 17Sulfolipids 1Others 0.54 7 15
a Total lipids as %, while lipid fractions are expressedas percent of the total lipids.

3.4 Phospho- and Glycolipids 179
Table 3.18. Occurrence of phosphatidyl derivates
Food Lipid P-containing Phosphatidyl derivativesb (mg/kg)(g/kg) lipidsa (g/kg) PC
PS PE PI
Milk 37.8 0.35 120 10 100 2Egg 113 35.1 27,000 ¾ 5810 ¾Meat (beef) 19 8.3 4290 690 1970 ¾Meat (chicken) 62 6.6 3320 850 1590 ¾Tuna fish 155 19.4 6410 1940 5030 ¾Potato 1.1 0.56 280 10 160 90Rice 6.2 0.89 320 30 350 ¾Soybean 183 17.8 7980 ¾ 4660 2500
a Phosphatidyl derivatives and other P-containing lipids, e. g., plasmalogens, sphingomyelins.b The abbreviations correspond to Formulas 3.26 – 3.29.
OH-group of choline, PC):
(3.26)
Phosphatidyl serine (phosphate group esterifiedwith the HO-group of the amino acid serine, PS):
(3.27)
Phosphatidyl ethanolamine (phosphate group es-terified with ethanolamine, PE):
(3.28)
Phosphatidyl inositol (phosphate group esterifiedwith inositol, PI):
(3.29)
A mixture of phosphatidyl serine and phos-phatidyl ethanolamine was once referred to ascephalin.
Examples of foods which contain phosphatidylderivatives are shown in Table 3.18. The differ-ences to the data in Table 3.17 are caused by thebiological range of variations.Only one acyl residue is cleaved by hydrolysis(cf. 3.7.1.2.1) with phospholipase A. This yieldsthe corresponding lyso-compounds from lecithinor phosphatidyl ethanolamine. Some of theselyso-derivatives occur in nature, e. g., in cereals.Phosphatidyl glycerol is invariably found in greenplants, particularly in chloroplasts:
L-α-Phosphatidyl-D-glycerol (3.30)
Cardiolipin, first identified in beef heart, is alsoa minor constituent of green plant lipids. Itschemical structure is diphosphatidyl glycerol:
Diphosphatidyl glycerol (cardiolipin) (3.31)
The plasmalogens occupy a special place in theclass of phospho-glycerides. They are phos-phatides in which position 1 of glycerol is linkedto a straight-chain aldehyde with 16 or 18 car-bons. The linkage is an enolether type with a dou-

180 3 Lipids
ble bond in the cis-configuration. Plasmalogensof 1-O-(1-alkenyl)-2-O-acylglycerophospholipidtype occur in small amounts in animal muscletissue and also in milk fat. The enol-ether linkage,unlike the ether bonds of a 1-O-alkylglycerol(cf. 3.6.2), is readily hydrolyzed even by weakacids.
Plasmalogen (3.32)
Phospholipids are sensitive to autoxidation sincethey contain an abundance of linoleic acid. Theother acid commonly present is palmitic acid.Phospholipids are soluble in chloroform-methanol and poorly soluble in water-freeacetone. The pKs value of the phosphate group isbetween 1 and 2. Phosphatidyl choline and phos-phatidyl ethanolamine are zwitter-ions at pH 7.Phospholipids can be hydrolyzed stepwise byalcoholic KOH. Under mild conditions, onlythe fatty acids are cleaved, whereas, with strongalkalies, the base moiety is released. The bondsbetween phosphoric acid and glycerol or phos-phoric acid and inositol are stable to alkalies, butare readily hydrolyzed by acids. Phosphatidylderivatives, together with triacylglycerols andsterols, occur in the lipid fraction of lipoproteins(cf. 3.5.1).
Lecithin. Lecithin plays a significant role asa surface-active agent in the production ofemulsions. “Raw lecithin”, especially that ofsoya and that isolated from egg yolk, is availablefor use on a commercial scale. “Raw lecithins”are complex mixtures of lipids with phosphatidylcholines, ethanolamines and inositols as maincomponents (Table 3.19).The major phospholipids of raw soya lecithin aregiven in Table 3.19. The manufacturer often sepa-rates lecithin into ethanol-soluble and ethanol-insoluble fractions.Pure lecithin is a W/O emulsifier with a HLB-value (cf. 8.15.2.3) of about 3. The HLB-valuerises to 8–11 by hydrolysis of lecithin tolysophosphatidyl choline; an o/w emulsifieris formed. Since the commercial lecithins arecomplex mixtures of lipids their HBL-valueslie within a wide range. The ethanol-insoluble
Table 3.19. Composition of glycerol phospholipids insoya “raw lecithin” and in the resulting fractionsa
Unfrac- Ethanol Ethanoltionated soluble insoluble
fraction fraction
Phosphatidylethanolamine 13–17 16.3 13.3
Phosphatidylcholine 20–27 49 6.6
Phosphatidylinositol 9 1 15.2
a Values in weight%.
fraction (Table 3.19) is suitable for stabilizationof W/O emulsions and the ethanol-solublefraction for O/W emulsions. To increase theHLB-value, “hydroxylated lecithins” are pro-duced by hydroxylation of the unsaturated acylgroups with hydrogen peroxide in the presenceof lactic-, citric- and tartaric acid.
3.4.1.2 Glyceroglycolipids
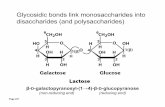
These lipids consist of 1,2-diacylglycerols anda mono-, di- or, less frequently, tri- or tetrasac-charide bound in position 3 of glycerol. Galactoseis predominant as the sugar component amongplant glycerolipids. The glyceroglycolipids oc-curring in wheat influence the baking properties(cf. 15.2.5).
(3.33)
Monogalactosyl diacylglycerol (MGDG)(1,2-diacyl-3-β-D-galactopyranosyl-L-glycerol)
(3.34)
Digalactosyl diacylglycerol (DGDG)

3.4 Phospho- and Glycolipids 181
(1,2-diacyl-3-(α-D-galactopyranosyl-1,6-β-D-ga-lactopyranosyl)-L-glycerol)6-O-acyl-MGDG and 6-0-acyl-DGDG are minorcomponents of plant lipids.Sulfolipids are glyceroglycolipids which arehighly soluble in water since they contain a sugarmoiety esterified with sulfuric acid. The sugarmoiety is 6-sulfochinovose. Sulfolipids occurin chloroplasts but are also detected in potatotubers:
(3.35)
Sulfolipid(1,2-diacyl-(6-sulfo-α-D-chinovosyl-1,3)-L-glycerol)
3.4.1.3 Sphingolipids
Sphingolipids contain sphingosine, an aminoalcohol with a long unsaturated hydrocarbonchain (D-erythro-1,3-dihydroxy-2-amino-trans-4-octadecene) instead of glycerol:
(3.36)
Sphingolipids which occur in plants, e. g., wheat,contain phytosphingosines:
(3.37)
The amino group in sphingolipids is linked toa fatty acid to form a carboxy amide, denoted asceramide. The primary hydroxyl group is eitheresterified with phosphoric acid (sphingophos-pholipid: ceramide-phosphate-base) or boundglycosidically to a mono- di-, or oligosaccharide
(sphingoglycolipid: ceramide-phosphate-sugarn ).In the third group of sphingolipids the ceramidemoiety is linked by a phosphate residue to thecarbohydrate building blocks. These compoundsare also referred to as phytoglycolipids.
Sphingophospholipids. Sphingomyelin is one ex-ample of a sphingophospholipid. It is the mostabundant sphingolipid and is found in myelin, thefatty substance of the sheath around nerve fibers.The structure of sphingomyelin is:
(3.38)
Sphingoglycolipids are found in tissue of ani-mal origin, milk and in plants (especially cere-als). Based on structural properties of the carbo-hydrate building blocks, one differentiates neutraland acid glycosphingolipids. The sulfatides andgangliosides also belong to this group.Lactosylceramide in milk and the ceramideglycosides of wheat are examples of neutralglycosphingolipids that contain, next to glucoseand mannose, also saturated (14:0–28:0) andmonounsaturated (16:1–26:1) 2-hydroxy- or2,3-dihydroxy fatty acids.Formula 3.40 a depicts a sphingoglycolipid ofwheat.Gangliosides contain sialic acid (N-acetyl-neuraminic acid; cf. Formula 3.40 b). In theganglioside fraction of milk monosialosyl-lactosyl-ceramide (cf. Formula 3.41) wasidentified.
Phytosphingolipids. These lipids also have a com-plex structure. Total hydrolysis yields phytosph-ingosine, inositol, phosphoric acid and variousmonosaccharides (galactose, arabinose, mannose,glucosamine, glucuronic acid).
(3.39)

182 3 Lipids
(3.40a)
(3.40b)
(3.41)
(3.42)
Phytosphingolipids are isolated from soya andpeanuts (cf. Formula 3.42).
3.4.2 Analysis
3.4.2.1 Extraction, Removal of Nonlipids
A solvent mixture of chloroform/methanol(2 + 1 v/v) is suitable for a quantitative extrac-tion of lipids. Addition of a small amount ofBHA (cf. 3.7.3.2.2) is recommended for thestabilization of lipids against autoxidation.Nonlipid impurities were earlier removed byshaking the extracts with a special salt solutionunder rather demanding conditions. An improvedprocedure, by which emulsion formation isavoided, is based on column chromatographywith dextran gels.
3.4.2.2 Separation and Identificationof Classes of Components
Isolated and purified lipids can be separatedinto classes by thin layer chromatography usingdeveloping solvents of different polarity. Fig-ure 3.12 shows an example of the separation ofneutral and polar lipids. Identification of polarlipids is based on using spraying reagents whichreact on the plates with the polar moiety ofthe lipid molecules. For example, phosphoricacid is identified by the molybdenum-bluereaction, monosaccharides by orcinol-FeCl3,choline by bismuth iodide (Dragendorff reagent),ethanolamine and serine by the ninhydrin reac-tion, and sphingosine by a chlorine-benzidinereagent.When sufficient material is available, it is advis-able to perform a preliminary separation of lipidsby column chromatography on magnesium sili-

3.5 Lipoproteins, Membranes 183
Fig. 3.11. HPLC-analysis of soy “raw lethicin”(according to Sotirhos et al. 1986) 1 Triacylglyc-erols, 2 free fatty acids, 3 phosphatidyl glycerol,4 cerebrosides, 5 phytosphingosine, 6 diphosphatidylglycerol, 7 digalactosyldiacyl glycerol, 8 phosphatidylethanolamine, 9 phosphatidyl inositol, 10 lysophos-phatidyl ethanolamine, 11 phosphatidic acid, 12 phos-phatidyl serine, 13 phosphatidyl choline, 14 lysophos-phatidyl choline
cate (florisil), silicic acid, hydrophobic dextrangel or a cellulose-based ion-exchanger, such asDEAE-cellulose.Also the HPLC-analysis of phospho- and gly-colipids is of growing importance. Figure 3.11for example demonstrates the separation of soya“raw lecithin”.
3.4.2.3 Analysis of Lipid Components
Fatty acid composition is determined aftermethanolysis of the lipid. For positional analysisof acyl residues (positions 1 or 2 in glycerol),phosphatidyl derivatives are selectively hy-drolyzed with phospholipases (cf. 3.7.1.2.1) andthe fatty acids liberated are analyzed by gaschromatography.The sphingosine base can also be determinedby gas chromatography after trimethylsilylderivatization. The length of the carbon skele-ton, of interest for phytosphingosine, can bedetermined by analyzing the aldehydes re-leased after the chain has been cleaved byperiodate:
(3.43)
The monosaccharides in glycolipids can alsobe determined by gas chromatography. Thelipids are hydrolyzed with trifluoroaceticacid and then derivatized to an acetylatedglyconic acid nitrile. By using this sugarderivative, the chromatogram is simplifiedbecause of the absence of sugar anomers(cf. 4.2.4.6).