Wave Nature of Matter - Linville · · 2011-03-12• de Broglie’s theory of matter waves can be...
Transcript of Wave Nature of Matter - Linville · · 2011-03-12• de Broglie’s theory of matter waves can be...

Wave Nature
of Matter

Wave-Particle Duality
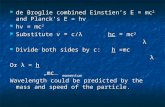
• de Broglie proposed that particles with momentum could have an associated wavelength (converse of photons having momentum)

• de Broglie wavelength
• only small, high speed particles will show wave characteristics (diffraction, interference)
λλ hp or ==
ph

• normal sized objects have wavelengths that are too small to be noticed, but for elementary particles, the wavelengths are the size of nuclei

Example
• A 0.15 kg baseball is moving at 40 m/s. Determine its de Broglie wavelength

Solution
msmkgsJ
ph
34101.1/4015.0
34-106.63
−×=
ו×=
=
λ
λ
λ • Atomic diameter: 10-10 m
• Nuclear diameter: 10-14 m

Example
• An electron is accelerated from rest by a potential difference of 1.50 kV. Determine the de Broglie wavelength of the electron.

Solution

Evidence
• Davisson and Germer reflected a beam of electrons off a crystal and obtained a series of maxima and minima patterns (just like a beam of light would give a series of light and dark bands when it passed through a diffraction grating)


Thomson
• G.P. Thomson detected electron diffraction patterns by passing a beam of electrons through a metal foil and obtaining an interference pattern


X-ray beam through Al foil
Electron beam through Al foil

• The Nobel Prize in Physics 1937 was awarded jointly to Davisson and Thomson "for their experimental discovery of the diffraction of electrons by crystals"

Significance of the de Broglie Hypothesis
• extended Einstein's work to relate the observed wavelength of matter to its momentum
• showed that wave particle duality was not only a strange behavior of light, but was a fundamental principle exhibited by both radiation and matter.

Back to Bohr…
• de Broglie’s theory of matter waves can be used to explain why electrons did not radiate in the Bohr model
• de Broglie viewed the electron as a standing wave
• the wavelength would be longer in higher energy levels because the electron speed is lower

• the circumference of an allowed orbit would be a whole number of electron wavelengths

• if a whole-number of wave lengths don’t fit the circumference, the result is destructive interference

Classical view of electrons (electrons are particles)
Lots of electrons pass through top opening
Lots of electrons pass through bottom opening
No electrons land directly on centre of screen

Electron diffraction
• When electrons are fired through 2 slits, a diffraction pattern forms whether electrons are fired one-by-one or in a continuous stream

Single electron events built up from an interference pattern in the double-slit experiment.

Quantum Mechanical Model
• Quantum mechanics combines the particle-wave nature of matter and energy into a single theory

• according to quantum mechanics, electrons do not exist in well defined orbits as proposed by Bohr
• quantum mechanics refer to orbitals

• the theory can only predict the probability of finding an electron in a certain region of space around the nucleus due to the wave nature of matter

Electron Cloud
• the region where the probability of finding an electron is high

• Bohr’s idea of allowed energy states still applies
• An electron will have 1 of the allowed energy states, but the state doesn’t correspond to a particular orbit

Distance from nucleus

• Higher energy states means a higher probability of finding the electron further from the nucleus

Wavefunction
• The wave function represents the probability of finding a given particle at a given point.
• These probability equations can diffract, interfere, and exhibit other wave-like properties

Compare and contrast: waves & particles
Waves• Extended in space
• Continuous
• Obey wave equations
• Diffract and interfere
• Have amplitude, wavelength, frequency & velocity
Particles• Points
• Discontinuous
• Obey equations of mechanics
• Collide and bounce
• Mass, size, velocity, momentum

STS
• Electron microscopes use the wave nature of electrons to produce magnifications much larger than with optical microscopes
• The wavelengths are about ten thousand times smaller than that of light

• Electrons are accelerated from the electron gun to the anode.
• The magnetic lens exerts a force on electrons; they spiral and become focused
• Scanning coils deflect the beam of electrons back and forth across the specimen


Detecting Gravity Waves
• Gravitational waves are ripples in the fabric of space and time by the collision of two black holes or by the cores of supernova explosions.

Detecting Gravity Waves
• Gravity waves will compress space in 1 direction and stretch it in the other. Light is split into two beams

Detecting Gravity Waves
• If the lengths of both arms remain unchanged, the 2 combining laser beams will cancel each other out

Detecting Gravity Waves
• If a gravity wave changed the length of the arms, the light beams would not cancel out, changing the interference pattern