Wave Mechanics – Classical Systems Copyright © 2011 Pearson Canada Inc. General Chemistry:...
-
Upload
peter-randolph-wade -
Category
Documents
-
view
220 -
download
1
Transcript of Wave Mechanics – Classical Systems Copyright © 2011 Pearson Canada Inc. General Chemistry:...

Wave Mechanics – Classical Systems
Copyright © 2011 Pearson Canada Inc. General Chemistry: Chapter 8 Slide 1 of 50
FIGURE 8-18
•Standing waves in a string
2Ln
Standing waves.Nodes do not undergo
displacement.
λ = , n = 1, 2, 3…

Dihydrogen Oxide Waves

Quantized Energies and Wave Functions
• Line spectra tell us that atomic (and molecular) energies are quantized. The switch from a classical description of objects to a quantum mechanical description becomes necessary as we move to physically smaller and lighter objects. In many cases these smaller particles are confined to move in confined regions of space (e.g. an electron in a H atom!).

Particle in a Box Model
• We live in a three dimensional world and would expect the wave functions used to describe atomic and molecular systems to have three spatial coordinates and time. We could write Ψ(x,y,z,t) and Ψ(r,ϴ,φ,t) for wave functions. In some cases the properties and energies of an atom do not change with time and we can write suitable (time independent) wave functions as Ψ(x,y,z) or Ψ(r,ϴ,φ).

Particle in a Box (One Dimension)
• Energy units (1 joule = 1 kg∙m2∙s-2) suggest that second order differential equations are needed to treat atomic and molecular energies. Advanced mathematics allows us to solve 2nd order differential equations and determine (a) the functional form of wave functions for particular objects/systems and (b) values for properties such as atomic energies.

Wave and Quantum Mechanics• We leave the detailed mathematics to higher
level courses. To begin, we consider an approximate wave function that is a model for some problems in chemistry and physics – the so-called particle in a box (PIAB) model. This crude model does provide insight into the atomic/molecular world. One dimensional, two dimensional and three dimensional PIAB models are available. We will consider the one dimensional PIAB model first.

PIAB – One Dimension• The wave functions for that we require are
found by solving the so-called Schrodinger equation, H ψn(x) = Eψn(x). This 2nd order differential equation can be tackled fairly simply (2nd year). It enables us to calculate allowed energy values, positions of nodes (zero probability of finding a particle) and where a particle is most likely to be found.

PIAB – One Dimension
• The wave function(s) on the next slide are written as Ψn(x) rather to emphasize that we have different wave functions for various values of the single quantum number, n. The single quantum number is a direct consequence of the use of a one dimensional PIAB model. For energies we have the result
• En = n2h2 where h is Planck’s constant.
• 8mL2

Particle in Box: Standing Waves, Quantum Particles, and Wave Functions
Copyright © 2011 Pearson Canada Inc. General Chemistry: Chapter 8 Slide 9 of 50
FIGURE 8-20
•The standing waves of a particle in a one-dimensional box
ψ, psi, the wave function.Should correspond to a standing
wave within the boundary of the system being described.
Particle in a box. Number of nodes increases as n and energy increase. Compare to Slide 1?

PIAB - Notes on the Ψn’s
• The PIAB wave functions exhibit nodes. As we move to higher energy (higher n) states the number of nodes increases. As well, as one moves to higher n values the characteristic wavelength decreases. (This is reminiscent of light where, again, the energy of a photon increases as the wavelength of the light decreases).The wave functions can have both positive and negative amplitude.

Amplitudes and Probabilities
• In quantum mechanics the probability of finding a particle at a particular point in space is proportional to the square of the amplitude of the wave function at that particular point. Probabilities must always be positive and the squares of the amplitudes for the first few one dimensional PIAB wave functions are shown on the next slide.

Copyright © 2011 Pearson Canada Inc. General Chemistry: Chapter 8 Slide 12 of 50
The probabilities of a particle in a one-dimensional boxFIGURE 8-21

Probabilities of Finding an e- ?• The graphs on the previous slide describe the
probability of finding a particle (electrons are especially of interest in chemistry) in different parts of the “box”. In all cases there is a 50% probability of finding a particle in the left half of the box. Calculus (or a graphing calculator) can tell us the probability of finding the particle in any arbitrarily chosen part of the box.

Probabilities – Class Examples• We will use Slide 12 to determine when
calculus must be used to calculate the probability of finding a particle in a given part of the box and when simpler “symmetry arguments” can be used. We’ll consider a number of cases where symmetry arguments can be used to specify exactly the probability of finding a particle in a certain part of the box.

Copyright 2011 Pearson Canada Inc. 8 - 15

PIAB Energies – Mass & Particle Size
• The PIAB model allows us to calculate energies for a particle in a particular quantum state as a function of quantum number (n), particle mass (m) and box length (L). As values of n increase energies increase. As particle mass increase and/or box size increases energy level spacings decrease rapidly. For the limiting case as m → and L → the energies vary continuously (familiar ground?).

PIAB – 3 Dimensions• The Schrodinger Eqtn. for the 3-dimensional
PIAB can also be solved.
• E = { + + }• Here a, b and c are the lengths of the 3 sides of
the box and n1, n2 and n3 are a set of three quantum numbers needed for this higher dimensionality case. We will need three quantum numbers when we discuss atoms!

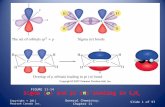
Spherical Polar Coordinates
• When we move from the one dimensional PIAB model to the wave functions for an electron in a H atom it’s simplest to use spherical polar coordinates when we construct the H atom wave functions. The correspondence between Cartesian and spherical polar coordinates is shown on the next slide.

Wave Functions of the Hydrogen Atom
Copyright © 2011 Pearson Canada Inc. General Chemistry: Chapter 8 Slide 19 of 50
FIGURE 8-22
•The relationship between spherical polar coordinates and Cartesian coordinates
Schrödinger, 1927 Eψ = H ψ
H (x,y,z) or H (r,θ,φ)
ψ(r,θ,φ) = R(r) Y(θ,φ)
R(r) is the radial wave function.
Y(θ,φ) is the angular wave function.

H Atom Wave Functions• The previous slide states that the H atom wave
functions, determined again by solving the Schrodinger equation, can be factored into an angular and a radial part if we employ spherical polar coordinates. The use of these coordinates makes it especially easy to locate nodes (regions of zero “electron density”) and to represent 3 dimensional probabilities (i.e. represent in 3 dimensions the probability of finding an electron in space/in an atom).

Sample Test 1 Examples
• A1. A 6.60 L tank containing oxygen gas at a temperature of 46ºC is cooled at constant volume to a final temperature of –10ºC and a pressure of 88.8 kPa. What was the initial pressure of the oxygen gas?
•

Sample Test 1 Examples
• A3. Calculate the value of ΔU in joules associated with the compression of a gas from 17.8 L to 12.9 L by a constant external pressure of 4.50 bar if, at the same time, the gas releases 1,225 joules of heat to the surroundings.

Sample Test 1 Examples
• A4. Write the balanced thermochemical equation corresponding to the molar enthalpy (heat) of formation of glucose, C6H12O6(s).
• •

Sample Test 1 Examples
• B1. Calculate the standard enthalpy change ∆Hº for the reaction below:•
• N2O(g) + NO2(g) 3 NO(g)
• • Data:•
• N2(g) + O2(g) 2 NO(g) ∆Hº = +180.7 kJ
•
• 2 NO(g) + O2(g) 2 NO2(g) ∆Hº = –113.1 kJ
•
• 2 N2O(g) 2 N2(g) + O2(g) ∆Hº = –163.2 kJ
• • • •