Wave Functions for Hydrogen Quantum Numbers ψ H...
Transcript of Wave Functions for Hydrogen Quantum Numbers ψ H...

1
Wave Functions for Hydrogen
• Eψ = H ψ
– H (x,y,z) or H (r,θ,φ)
eH
22
2
ψ(r,θ,φ) = R(r) Y(θ,φ)
R(r) is the radial wave function.
Y(θ,φ) is the angular wave function.
rH
o 42
Quantum Numbers
• When solving the Schrödinger equation, three quantum numbers are used.
– Principal quantum number, n (n = 1, 2, 3, 4, 5, …)
– Secondary quantum number, l
– Magnetic quantum number, ml
Principle Shells and Subshells
• Principle electronic shell, n = 1, 2, 3…– Orbital size
• Angular momentum quantum number,l = 0, 1, 2…(n-1)– Orbital shape
l = 0, sl = 1, pl = 2, dl = 3, f
• Magnetic quantum number, – Orbital orientation– ml= -l …-2, -1, 0, 1, 2…+ l
Quantum Numbers
• Note the relationship between number of orbitals within s, p, d, and f and ml. Each principle shell contains n² orbitals.
Orbital Energies for 1 Electron Atom
atomBohrtheforassamenam
Ee
n 220
2
2
Interp
reting an
d R
Orb
itals of the H R
epresen
ting th
e H
ydrogen
Atom
.

2
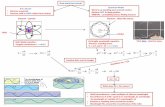
Visualizing Orbitals
• Wave functions for the first five orbitals of hydrogen.
• The wave function is written in spherical polar coordinates with the nucleus at the originwith the nucleus at the origin.
– Point in space defined by radiusr, and angles and .
– r determines the orbital size.
– and determine the orbital shape.
Visualizing Orbitals
• A point in space defined by radius r, and angles and .
• Chemists usually think of orbitals in terms of pictures.
– The space occupied by an orbital is a 90% probability of finding an electron.
– A plot of the angular part of the wave function gives the shape of the corresponding orbital.
Visualizing Orbitals
• An orbital depicts the probability of finding an electron at a given location.– Node is a location where the electron is never found (ψ = 0)
• The radial part of the wave function describes how the probability of finding an electron varies with distance from the nucleus.the nucleus.– Spherical nodes are generated by the radial portion of the wave
function, R(r).
• The angular part of the wave function describes how the probability of finding an electron varies with orientation from the nucleus.– Nodal nodes are generated by the angular portion of the wave
function, Y(θ,φ) .
Visualizing Orbitals
• s orbitals are spherical
• p orbitals have two lobes separated by a nodal plane.– A nodal plane is a plane where the probability of finding an electron is zero
(here the yz plane).
• d orbitals have more complicated shapes due to the presence of two nodal planes.
Probability Distribution for the s Orbitals
No spherical nodes 2 spherical nodes1 spherical node
Radial Probability Distribution for 1s Orbital

3
p Orbitals
Nodal Plane
The Boundary Surface Representations of All Three 2p Orbitals
The Boundary Surfaces of All of the 3d OrbitalsRepresentation of the 4f Orbitals in Terms
of Their Boundary Surfaces
Orbital Nodes
An orbital is description by the quantum numbers n, l and ml
• Total number nodes (excluding r = 0 and ) is n-1
• The number of angular nodes is l whose location depends on the value of ml
• The number of spherical nodes is n-1- l
So for a 4d orbital (n=4, l =2)
Would have 3 (n-1=3) total nodes; of which 2 (l =2) are angular nodes and 1 (n-1- l =1) is spherical node
1 Electron Atomic Orbital Example
Which is larger?
• the H 2p orbital or the H 3p orbital
• the H 1s orbital or the Li2+ 1s orbital
Which is lower in energy?
• the H 2p orbital or the H 3p orbital
• a H 4s orbital or a H 4f orbital
How many nodes and where are they located in a
• 4s orbital
• 3py orbital
• 4dxz orbital

4
Electron Spin: A Fourth Quantum Number
• The spin quantum number, ms, determines the number of electrons that can occupy an orbital.– ms = ±1/2– Electrons described as “spin up” or “spin down”.– An electron is specified by a set of four quantum numbers.
The Pauli Exclusion Principle
The Pauli Exclusion Principle states:• No two electrons in an atom can have the same four
quantum numbers (n, l, ml, ms).
– Two electrons can have the same values of n, l, and ml, but different values of ms.
– Two electrons maximum per orbital.
– Two electrons occupying the same orbital are spin paired.
Multi-electron Atoms
• Hydrogen wavefunctions are for only one e-
species.
• Electron-electron repulsion in multi-electron atoms.– Use Hydrogen-like orbitals (by approximation).
Orbital Energies and Electron Configurations
• For electrons in larger orbitals, the charge “felt” is a combination of the actual nuclear charge and the offsetting charge of electrons in lower orbitals.
– The masking of the nuclear charge is called shielding.
– Shielding results in a reduced, effective nuclear charge.
Penetration and Shielding
Zeff is the effective nuclear charge.
A Comparison of the Radial Probability Distributions of the 2s and 2p Orbitals
• Effective nuclear charge allows for understanding of the energy differences between orbitals.– 2s orbital: the small local
maximum close to the l l inucleus results in an
electron with a higher effective nuclear charge.
– 2p orbital: lacks the local minimum close to the nucleus of the 2s orbital.
• Lower effective nuclear charge for 2p electrons.

5
The Radial Probability Distribution of the 3s Orbital A Comparison of the Radial Probability Distributions of the 3s, 3p, and 3d Orbitals
The Energies of Orbitals in Polyelectronic AtomsOrbital Energies
Aufbau Process
As we add protons element by element across the periodic table, electrons are similarly added into hydrogen-like orbitals, starting with the lower-energy orbitals and building up.
We can determine the arrangement of electrons (how many electrons in each orbital), called the electron configuration, for all elements on the Periodic Table.
We follow two “rules” to do this1. We saw that the Pauli Exclusion Principle states that no two
electrons can have the same four quantum numbers…so only two electrons can be added to each orbital.
2. Hund’s Rule states that the lowest energy arrangement of electrons is the one with the maximum number of unpaired electrons (but still following the Pauli Exclusion Principle!)
Orbital Diagram
• A notation that shows how many electrons an atom has in each of its occupied electron orbitals.
O 1 22 22 4Oxygen: 1s22s22p4
Oxygen: 1s 2s 2p

6
Orbital Filling Ground State Electron ConfigurationsZ Atom Configuration 1s 2s 2p
1
2
3
4
5
6
7
8
9
10
Filling the d OrbitalsHund’s Rule and the Aufbau Principle
• The inner electrons, which lie closer to the nucleus, are referred to as core electrons.
– Core electrons can be represented by the noble gas with the same electronic configuration.
• The outer electrons are usually referred to as valence electrons.
– Valence electrons are shown explicitly when a noble gas shorthand is used to write electronic configurations.
– Valence electrons determine reactivity.
Electron Configurations of Some Groups of ElementsThe Periodic Table and Electron Configurations
• The periodic table and the electronic configurations predicted by quantum mechanics are related.
– The periodic table is broken into s, p, d, and f blocks.
– Elements in each block have the same subshell for the highest electron.
– Structure of periodic table can be used to predict electronic configurations.

7
The Periodic Table and Electron Configurations
• The shape of the periodic table can be broken down into blocks according to the type of orbital occupied by the highest energy electron in the ground state.
• We find the element of interest in the periodic table and write its core electrons using the shorthand notation with the previous rare gas element. Then we determine the valence electrons by noting where the element sits within its own period in the table.
Electron Configurations Example
• For the following chemical species, what are the expected electron configurations.– Ca
– Fe
– Eu
• What atom has the following electron configurations?– 1s2 2s2 2p6 3s2 3p5
– 1s2 2s2 2p6 3s2 3p6 3d7 4s2