· Web viewHydrogenation of Dibenzo-18-crown-6 ether using γ-Al 2 O 3 supportedRu-Pd and Ru-Ni...
Transcript of · Web viewHydrogenation of Dibenzo-18-crown-6 ether using γ-Al 2 O 3 supportedRu-Pd and Ru-Ni...

Hydrogenation of Dibenzo-18-crown-6 ether using γ-Al2
O3
supported
Ru-Pd and Ru-Ni bimetallic nanoalloy catalysts
Yogeshwar R. Suryawanshia,b, Mousumi Chakrabortya,*, Smita Jauharib,
Sulekha Mukhopadhyayc, K. T. Shenoyc,
aChemical Engineering Department & bApplied Chemistry Department,
S.V. National Institute of Technology,
Surat-395 007, Gujarat, India
&
cChemical Engineering Division &
Bhabha Atomic Research Centre (BARC),
Mumbai- 400085, Maharashtra, India
ABSTRACT:
Ruthenium-palladium (Ru-Pd) and ruthenium-nickel (Ru-Ni) bimetallic nanoalloy particles with various metal compositions were synthesized by microwave
irradiated (MWI) solvothermal technique using PVP (poly-N-vinyl-2-pyrrolidone) as capping agent and ethylene glycol as a solvent as well as reducing agent.
Synthesized bimetallic nanoalloyparticles were subsequently impregenated onto γ-Al2O
3 support to obtain supported nanoalloy catalysts. Agglomeration of
nanoalloyparticles were restricted by excess solvent, simultaneously distilled and recovered after completion of reaction. Synthesized bimetallic nanoalloy
catalysts were used for the hydrogenation of Dibenzo-18-crown-6 ether (DB18C6) at 9 MPa, 120oC temperature and 3.5 h. It was observed that bimetallic
nanolloyscatalyst synthesized by MWI using Ru:Pd 3:1% (w/w), exhibited higher catalytic activity and resulted 98.9% conversion of DB18C6 with 100%
selectivity towards cis-syn-cis dicyclohexano-18-crown-6 ether (CSC DCH18C6).
1. INTRODUCTION:

Crown ether compounds have been widely used for complexation and seperation of metal ions, phase transfer catalysis, host-guest chemistry and
supramolecular chemistry (Yamato et al. 2002; Landre et al. 1993). Beside other fission products, 90Sr and 137Cs are formed in nuclear reactors. The
medium activity water (MAW) produced by the PUREX process, contains a fraction of these two isotopes. Because of their long half-lives ( 137Cs: 30.1y,
90Sr: 28.5 y), their separation could reduce storage risks and costs of the solidified waste (Blasius et al. 1985). The possible application of crown ethers for
the recovery of radionuclides [Sr, Cs, transuranium elements] from real radioactive waste solutions using DCH18C6 and its derivatives have been reported
(Abashkin et al. 1996).
The hydrogenation of aromatic compounds is well-documented in the literature as far
as parent hydrocarbons are concerned, but is scarcer as regards the stereoselective
hydrogenation of substituted aromatics (Landre et al. 1994; Gao et al. 2012; Nandanwar
et al. 2013). DCH18C6 is usually produced by the catalytic hydrogenation of dibenzo-18-
crown-6 (DB18C6), which yields mainly two stereoisomers, cis-syn-cis-DCH18C6 and cis-
anti-cis-DCH18C6, while the cis-syn-cis isomer exhibits greater extractability and
selectivity for strontium than the cis-anti-cis isomer (Roucoux et al. 2002; Suryawanshi et
al. 2013). It is attractive to control the stereoselectivity of the catalytic hydrogenation,
controlling the steps involved and reaction mechanism of the synthesis process. Landre et
al. used colloidal Rh catalyst for the hydrogenation DB18C6 using phase transfer catalyst.
Recently, several researchers realized that ultrafine amorphous alloy particles could be
new catalytic materials that exhibit attractive selectivity and activity for hydrogenation
reactions (Zhu et al. 2015). Ruthenium nanocatalyst, Rh-Fe/MgO catalyst and Ru/γ-Al2O3
nanocatalyst were used for hydrogenation of crown ether compounds (Gao et al. 2012;
Salih et al. 2014; Suryawanshi et al. 2015). Bimetallic nanoscopic materials are that their
chemical and physical properties are quite different from those of bulk solids and those of
2

atoms. This phenomenon has been known as quantum effect (Chen et al. 2013). Bimetallic
alloy (solid solutions or intermetallics compounds) nanostructures, synthesized from two
single components, have been of interest because of their superior properties, in
comparison with their respective single-component species, that is, Ag, Pd, Rh, and so
forth (Ugalde et al. 2013). Many types of stable bimetallic particles have been reported,
such as platinum-copper, platinum-rhodium, palladium-nickel, platinum-cobalt, etc. The
attractiveness of such systems consists of a number of possible beneficial features,
as compared to monometallic ones i.e. improved selectivity and stability, reduced
side reactions, a better susceptibility to reactivation. The addition of the second metal
plays a key role in controlling the activity, selectivity, and stability of catalysts in certain
reaction. Ruthenium is usually combined with noble as well as non noble metals to
demonstrate the synergistic effect for its better activity test (Yu et al. 1997). However, very
little information is known about the electronic structures of bimetallic clusters of group 8-
10. Since both Ni and Pd belong to the same group 10 in the periodic table, it is interesting
to check their synergistic effect on their catalytic performance. Such effect on both nickel
and palladium combined with ruthenium-supported catalysts finds a wide application,
especially in different types of hydrogenation reactions. In recent years, MWI is employed
in the synthesis of several nanosized materials. It is demonstrated that MWI technique
allows uniform heating, thus producing more homogeneous nucleation sites in the
preparation of metal nanostructured particles. In this context, MWI application to the one-
step synthesis of bimetallic nanoalloy catalysts, and in particular of ruthenium-palladium
and ruthenium-nickel based systems, has been recently explored (Galletti et al. 2010).
3

So in light of the reported literature, bimetallic nanoalloy catalysts Ru-Pd and Ru-Ni
were synthesized by MWI technique, characterized and used to carry out hydrogenation of
DB18C6. Synergistic effect of Pd in Ru-Pd nanoalloy catalyst towards conversion and
stereoselectivity were also discussed herein.
2. EXPERIMENTAL & PROCEDURE
2.1. Materials
Ruthenium trichloride (RuCl3.nH2O, Ru Content ≥ 37%), Palladium chloride (PdCl2 ,Pd
content ≥ 60%), Nickel chloride (NiCl2.6H2O, Ni content ≥ 90%) was purchased from Merck
chemicals, India. Poly (N-vinyl-2-Pyrrolidone) (PVP as stabilizer, average molecular weight
40,000) was purchased from Heny fine chemicals, India. Other organic solvents like n-
BuOH, chloroform, acetone, ethylene glycol of analytical grade were purchased from
Merck chemicals, India and used without further purification. DB18C6 (Dibenzo-18-crown-
6 ether), γ-alumina between (100-200 mesh) and commercial Ru/γ-Al2O3 (5 % (w/w) were
purchased from Sigma Aldrich India.
2.2. Catalyst preparation
2.2.1. Ru-Pd and Ru-Ni nanoalloy catalysts:
1.44x10-5 moles of RuCl3.nH2O and 1.73X10-4 moles of PVP were added in 30ml of
ethylene glycol and stirred well as the solution get homogenised and precipitate free.
1.90X10-3 moles of PdCl2 and dil. 2-3 drop dil. HCl were taken in a beaker and heated up to
60oC for 2 min. 0.009325 moles of NiCl2.6H2O was taken in another beaker. Then
homogenised dark red wine solution (RuCl3+PVP+EG) and faint red solutions of
(PdCl2+EG) and (NiCl2+EG) then mixed separately and charged into vial (PTFE reactor).
4

MWI given at optimized (24 min and 300W, ~200oC) conditions (Suryawanshi et al. 2013).
The dark black solution of Ru-Pd and Ru-Ni nanocolloid were obtained. To prepare dry
Ru:Pd/γ-Al2O3 and Ru:Ni/γ-Al2O3 nanocatalyst, solutions were transferred onto the support,
re-dispersed in methanol, mechanically stirred (7500 rpm using Ultraturax) for 24 h,
washed with acetone to remove the organic and inorganic impurities and dried for 12-16 h
in a hot air vacuum on 220oC followed by calcination on 300oC for 10-12 h. The dark dry
catalysts then weighted and packed into dry vial and preserved in desiccator.
2.3. Catalyst characterization
Absorption spectra of Ru-Pd and Ru-Ni nanocolloids were recorded using UV˗vis
spectrophotometer (HACH, Germany). Nanoparticle size was obtained using dynamic light
scattering (Malvern Zetasizer, Nano ZS 90, U.K.). XRD analysis was performed on Bruker
D8 (AXE). Powder X-ray diffractometer with Cu-Kα radiation (λ=1.54056 Å) in the range of
5°-90°. TEM images were obtained with a Philips Tecnai - 20, Holland.
2.4. Catalyst activity measurement hydrogenation of DB18C6:
The hydrogenation of DB18C6 to DCH18C6 was carried out in a 100 mL stainless steel
autoclave reactor (Amar Technology, Mumbai. India). The reactor built with a
thermocouple, hydrogen inlet valve, pressure gauge and magnetic stirrer. 30 mL butanol, 3
gm of DB18C6 and known quantity (0.3 gm) of prepared activated (reduced in 1h
continuous flow of H2) catalyst was charged into the reactor at optimum conditions (9 MPa
H2 , 120oC temperature and 3.5 h) (Yu et al. 1997). After the desired reaction time, the
mixture was quickly cooled down and separated by filtration.
3. Results and Discussion
5

3.1.1. UV-vis absorption spectroscopy analysis:
For Ru-Pd nanocolloids, at the beginning, the reaction mixture showed two broad hump absorption peaks (Figure 1) around 370nm and 470nm, which
could be attributed to the charge transfer from the ligand of Cl - to Ru3+ and Pd2+ ions in solution. Under irradiation, the baseline uplifted due to plasmon
scattering of nanoparticles: this plasmon absorption with time declined, due to the increase of the nanoparticle size. After MWI process (24 mins) UV-vis
spectra, no significant absorption peak was found, indicating that all of the metal ions were reduced completely ( Hei et al. 2012; Berger et al. 2010; Rakap et
al. 2014)
Figure 1. UV-Vis spectra for Ru:Pd nanocolloids.
From figure 2 it was observed that broad hump absorption peak was obtained at 370nm collectively for Ru3+ and Ni2+ ions, as PVP failed to restrict
agglomeration of nanocolloids, which disaapeared after MWI process (24 min). Diminishing of absorption peak at 370nm confirmed the formation of
nanoparticles ( Hei et al. 2012; Rakap et al. 2014; Guo et al. 2013).
6

Figure 2. UV-Vis spectra for Ru:Ni nanocolloids.
3.1.2. Dynamic light scattering (DLS) analysis:
Bimetallic Ru-Pd and Ru:Ni nanocolloids with varying metal composition (3:1, 2:2, 1:3%
(w/w)) were analysed. The average particle size ~19nm was obtained for 3:1 Ru-Pd
composition which was found to increase to 21nm for 2:2 and 48nm for 1:3 compositions
(Figure 3).
Figure 3. Particle size distribution by DLS technique for Ru:Pd nanocolloids.
7

Same trend was observed in case of Ru:Ni nanocolloids. The average particle size was
found to increase from 50nm to 100nm (Figure 4). Actually with increase in metal
(comparatively higher amount, 0.009325 moles of nickel used for 3:1% (w/w))
concentration, the collision frequency of the formed particles increased and protection
obtained by adsorption of surfactant molecules on the particles surface was weakened due
to lower surfactant concentration compared to precursor concentration. Thus the tiny
particles agglomerated to form larger particles. TEM analysis also confirmed same trend.
Figure 4. Particle size distribution by DLS technique for Ru:Pd nanocolloids.
3.1.3. X-ray diffraction analysis (XRD):
The bimetallic nanoalloy catalyst of Ru-Pd/γ-Al2O3 (Figure 5) showed peaks at 2θ =
38.4° (100), 44.08° (101) for ruthenium metal particles (highlighted by Δ) of the crystal
structure (JCPDS file no. 06-0663) and peak at 2θ = 51.2°(012) corresponding to
palladium metal particles21 . Diffraction patterns are broad because of the small sizes of the
8

colloidal nanoalloy particles. The presence of support material γ-alumina was confirmed by
the characteristic peak at 2θ = 66.5° (highlighted by *) (JCPDS file No. 10-0425).
Figure 5. XRD pattern for Ru:Pd/γ-Al2O3 (3:1 % (w/w)) nanocatalyst.
The bimetallic sample of Ru-Ni/γ-Al2O3 (Figure 6) nanoalloy catalyst also showed peaks for
ruthenium metal particles at 2θ = 38.4° (100), 44.08° (101) (highlighted by Δ). Diffraction
peaks around 2θ = 52.4° (200), and 75.6° (220) showed FCC structure of nickel
(highlighted by •). The characteristic peak also confirmed the presence of support material
γ-alumina. The average particle size of the Ru-Pd/γ-Al2O3 and Ru-Ni/γ-Al2O3 nanoalloy
catalyst were calculated from the Scherrer equation and were found in to be of 13 nm, 28
nm respectively ( Rakap et al 2014; Haque et al. 2010).
9

Figure 6. XRD pattern for Ru:Ni/γ-Al2O3 (3:1 % (w/w)) nanocatalyst.
3.1.4. Transmission electron microscopy analysis (TEM):
Figures 7a & 8a showed that bimetallic nanoparticles imaged as black dark dots and
were uniformly dispersed on the γ-Al2O3 support, with size of the Ru-Pd and Ru-Ni (3:1%
(w/w)) nanoparticles ranging from 3-15nm & 15-30nm respectively. Average particle sizes
(~19nm for Ru-Pd & ~30nm for Ru-Ni) were in good agreement with the particle size
calculated by the Debye Scherrer formula (~13nm) and (~28nm) respectively.
10

Figure 7. TEM images for a) Ru:Pd/γ-Al2O3 (3:1 % (w/w)) nanocatalyst c) Particle size
distribution graph of Ru:Pd/γ-Al2O3.
Figure 8. TEM images for a) Ru:Ni/γ-Al2O3 (3:1 % (w/w)) nanocatalyst b) Particle size
distribution graph of Ru:Ni/γ-Al2O3.
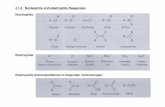
3.2. Catalytic activity towards DB18C6 hydrogenation reaction:
Hydrogenation of DB18C6 was carried out in the presence of continuous flow of H2 at
different time interval. Initially, the reaction pressure, temperature and time were optimized
to achieve maximum conversion and found to be 9 MPa, 393K temperature and 3.5 h.
(Scheme 1). Figure 9, concentration profile of DB18C6, showed that 80 to 90% of feed
(DB18C6) reacted within 50min of reaction time using Ru-Pd (3:1% (w/w)) nanoalloy
catalyst.
11

0 50 100 150 2000
0.10.20.30.40.50.60.70.80.9
1 Ru:Pd 3:1Ru:Pd 2:2
Time (min)
C/C
o
Figure 9. C/C0 versus time for hydrogenation of DB18C6 to DCH18C6.
Reaction conditions: temperature 100oC; pressure 9 MPa; catalyst amount 0.3 gm
(Ru:Pd 3-5% (w/w) Ru); speed of agitation 160 rpm; 3gm DB18C6 and 30 mL n-
Butanol (solvent).
Table 1 showed the effect of Ru-Pd loading [3:1, 2:2, 1:3% (w/w)] on DB18C6 conversion.
Synthesized product and pure CSC DCH18C6 curves were found identical in Liquid XRD
indicated that mainly CSC product formed in the reaction mixture (Figure 10). Product
analysis showed maximum 98.9% conversion with 100% selectivity towards CSC
DCH18C6 using 3:1% (w/w) Ru-Pd loaded nanoalloy catalyst, which indicated better result
in terms of selectivity than other nanoalloy catalyst.
12

Figure 10. Liquid XRD of synthesized product CSC DCH18C6.
Table 1: Effect of ruthenium loading on the conversion of DB18C6.
Entry Catalysts Conversion (%) Initial rate constant k (min-1)
1 *5% Ru/γ-Al2O3 94.0 0.725
2 #4% Ru/γ-Al2O3 96.5 0.977
3 3:1% Ru:Pd/γ-Al2O3
98.9 0.630
* 5% Ru/γ-Al2O3 Commercial catalyst
# 4% Ru/γ-Al2O3 Microwave Irradiated synthesized catalyst
With increasing Pd amount in Pd-Ru nanoalloy catalyst, particle size increased which
resulted lower surface area and lower conversion. Ru-Ni nanoalloy catalysts [3:1, 2:2,
1:3% (w/w)] failed to show any significant activity for the hydrogenation of DB18C6.
13

Suryawanshi et al. (2015) synthesized 4% (w/w) microwave irradiated (MWI) catalyst and
used for the above mentioned reaction. Catalytic activity of 3:1% (w/w) Ru-Pd, 4% (w/w)
microwave irradiated (MWI) and 5% (w/w) imported (IMP) nanocatalysts were compared. It
was observed from (Figure 11) that 3:1% (w/w) Ru-Pd showed highest conversion
although initial rate of reaction was slower compared to other two nanocatalysts. Actually
Ru-Pd nanocatalyst provided multiple and synergetic active sites (Ru0, Pd0) for the
hydrogenation of DB18C6 to DCH18C6. Therefore, hydrogen and benzene ring (in
DB18C6) would be adsorbed on the active sites of Ru via interaction of its pi-electrons with
Pd0 as “bridge” for transferring the activated H• species to the activated benzene ring via
spillover process (Li et al. 2006; Crisafulli et al. 1999). Gao et al. (2012) and Suryawanshi
et al. (2015) discussed formation mechanism of CSC DCH18C6. Actually higher density of
reduced ruthenium and palladium provided larger repulsion to the cyclohexyl ring, the first
reduced benzene ring of DB18C6 and thus pushed the cyclohexyl ring to the opposite side
of the catalyst surface and produced mainly CSC DCH18C6.
0 50 100 150 2000
0.2
0.4
0.6
0.8
1Ru:Pd 3:1
5 wt% Ru IMP
4 wt% Ru MWI
Time (min)
C/C
o
14

0 5 10 15 20 25 30 35 400
0.2
0.4
0.6
0.8
1Ru:Pd 3:15 wt% Ru IMP
Time (min)
C/C
o
Figure 11. C/C0 versus time for hydrogenation of DB18C6 to DCH18C6.
Reaction conditions: temperature 100oC; pressure 9 MPa; catalyst amount 0.3 gm (Ru:Pd
3:1% (w/w) Ru 4% (w/w)); speed of agitation 160 rpm; 3gm DB18C6 and 30 mL n-Butanol
(solvent).
This reaction could be considered as pseudo first order with respect to DB18C6 as
concentration of hydrogen will be more as compared to DB18C6.
[CA] = [CA0] e-kt ----------------------------------- (1)
Where, CA = concentration of the reactant, DB18C6 at time t, CA0 = initial concentration of
the reactant and k = rate constant for the pseudo first-order reaction
Rate constant for first-order reaction can be calculated by the equation
ln[CA/CA0] = -kt ------------------------------- (2)
It was found from Table 1 that the Ru:Pd 3:1% (w/w) loading showed the highest
conversion (98.9%) with (100%) selectivity towards CSC DCH18C6 but initial rate of
reaction was comparatively slower 0.63 min-1 than other two nanocatalysts.
15

4. Conclusion
Supported ruthenium palladium and ruthenium nickel metal bimetallic nanoalloy catalyst
were synthesized by microwave irradiated solvothermal technique. Synthesized bimetallic
nanoalloy catalyst activity was tested for the hydrogenation reaction of DB18C6 to
DCH18C6. Ru-Pd/γ-Al2O3 (3:1% (w/w)) exhibited smaller particle size, large active surface
area and higher conversion of DB18C6 (98.9%) with 100% selectivity towards CSC
DCH18C6, which was even better compared to 4% (w/w) microwave irradiated (MWI) and
5% (w/w) imported (IMP) nanocatalysts. Ru-Ni nanoalloy catalysts failed to show any
significant activity for the hydrogenation of DB18C6.
REFERENCES:
Abashkin, V.M. Wester, D.W. Campbell, J.A. and Grant, K.E., (1996), “Radiation stability of
cis-isomers of dicyclohexano-18-crown-6.” Radiat. Phys. Chem. Vol. 48, 463-472.
Berger, D. Traistaru, G.A. Vasile, B.S. Jitaru, I. and Matei, C. (2010), “Palladium
nanoparticles synthesis with controlled morphology obtained by polyol method.” U.
P. B. Sci. Bull. Vol. 72(1), 114-120.
Blasius, E. Klein, W. and Schon, U.J. (1985), “Seperation of strontinum from nuclear waste
solutions by solvent extraction with crown ethers.” Radioanal. Nucl. Chem. Vol. 89,
389-398.
Chen, Y.W. and Lee, D.S. (2013), “Selective hydrogenation of p-chloronitrobenzene on
nanosized PdNiB catalysts.” J. Nanoparticles Vol. 10, 1-10.
Crisafulli, C. Scire, S. Maggiore, R. Minico, S. and Galvagno. S. (1999), “CO2 reforming of
methane over Ni-Ru and Ni–Pd bimetallic catalysts.” Catal. Lett. Vol. 59, 21-26.
16

Galletti, A.M.R. Antonetti, C. Venezia, A.M. and Giambastiani, G. (2010), “An easy
microwave-assisted process for the synthesis of nanostructured palladium catalysts
and their use in the selective hydrogenation of cinnamaldehyde.” Appl. Catal., A,
Vol. 386, 124-131.
Gao, J. Chen, S. and Chen, J. (2012), “Stereoselective reduction of dibenzo-18-crown-6
ether to dicyclohexano-18-crown-6 ether catalyzed by ruthenium catalysts.” Catal.
Commun. Vol. 28, 27-31.
Guo, X. Zheng, F. Guo, M. Zhang, M. and Chou, K. (2013), “Preparation and uv property
of size-controlled monodisperse nickel nanoparticles (<10 nm) by reductive
method.” Rare. Met. Vol. 32, 179-185.
Haque, K.M.A. Hussain, M.S. Alam, S.S. and Islam, S.M.S. (2010), “Synthesis of nano-
nickel by a wet chemical reduction method in the presence of surfactant (SDS) and
a polymer (PVP).” Afri. J. Pure Appl. Chem. Vol. 4, 58-63.
Hei, H. He, H. Wang, R. Liu, X. and Zhang. G. (2012), “Controlled synthesis and
characterization of noble metal nanoparticles.” Soft. Nano.Lett. Vol 2, 34-40.
Landre, P.D. Lemaire M. Richard, D. and Gallezot, P. (1993), “A stereoselective reduction
of dibenzo-18-crown-6 ether to dicyclohexyl-18-crown-6 ether.” J. Mol. Catal. Vol.
78, 257-261.
Landre, P.D. Richard, D. Draye, M. Gallezot, P. and Lemaire, M. (1994), “Colloidal
rhodium: a new catalytic system for the reduction of dibenzo-18-crown-6 ether.” J.
Catal. Vol. 147, 214-222.
17

Li, D. and Komarneni, S. (2006), “Microwave-assisted polyol process for synthesis of Ni
nanoparticles.” J. Am. Ceram. Soc. Vol. 89, 1510.
Liu, M. Yu, W. Liu, H. Zheng, J. (1999), “Preparation and characterization of polymer-
stabilized ruthenium-platinum and ruthenium-palladium bimetallic colloids and their
catalytic properties for hydrogenation of o-chloronitrobenzene.” J. Colloid Interface
Sci. Vol. 214, 231-237.
Nandanwar, S.U. Chakraborty, M. Mukhopadhyay S. and Shenoy, K.T. (2013), “Benzene
hydrogenation over highly active monodisperse Ru/γ-Al2O3 nanocatalyst
synthesized by (w/o) reverse microemulsion.” Reac. Kinet. Mech. Cat. Vol. 108,
473-489.
Rakap, M. (2014), “Hydrolysis of sodium borohydride and ammonia borane for hydrogen
generation using highly efficient poly (n-vinyl-2-pyrrolidone)- stabilized Ru-Pd
nanoparticles as catalyst.” Int. J. Green Energy. Vol. 32, 1-45.
Roucoux, A. Schulz, J. and Patin., H. (2002), “Reduced transition metal colloids: a novel
family of reusable catalysts.” Chem. Rev. Vol. 102, 3757-3778.
Salih, A.A.M. Li, Y. Fan, J. Yi, C. and Yang, B. (2014), “Preparation of novel membrane
material 4’,4”(5”)-di-tert-butyldicyclohexyl-18-crown-6.” Advanced Material Research
Vol. 960-961, 73-77.
Suryawanshi, Y.R. Chakraborty, M. Jauhari, S. Mukhopadhyay, S. Shenoy, K.T. and
Shidharkrishna, R. (2013), “Microwave irradiation solvothermal technique: an
optimized protocol for size-control synthesis of Ru nanoparticles.” Cryst. Res.
Technol. Vol. 48(2), 69-74.
18

Suryawanshi, Y.R. Chakraborty, M. Jauhari, S. Mukhopadhyay, S. Shenoy, K.T. and Sen,
D. (2015), “Selective hydrogenation of dibenzo-18-crown-6 ether over highly active
monodisperse Ru/-Al2O3 nanocatalyst.” Bulletin of Chemical Reaction Engineering
& Catalysis. Vol.10(1), 23-29.
Ugalde, M. Chavira, E. Ochoa-Lara, M.T. Figueroa, I.A. Quintanar, C. and Tejeda. A.
(2013), “Synthesis by microwaves of bimetallic nano-rhodium-palladium.” J.
Nanotechnology. 1-9.
Yamato, K. Bartsch, R.A. Dietz, M.L. and Rogers, R.D. (2002), “Improved stereospecific
synthesis of the trans-isomers of dicyclohexano-18-crown-6 and the solid-state
structure of the trans–syn-trans-isomer.” Tetrahedron Lett. Vol. 43, 2153-2156.
Yu, Z. Liao, S. Xu, Y. Yang, B. and Yu, D. (1997), “Hydrogenation of nitroaromatics by
polymer-anchored bimetallic palladium-ruthenium and palladium-platinum catalysts
under mild conditions.” J. Mol. Catal., A: Chem. Vol. 120, 247-255.
Zhu, L. Sun, H. Fu, H. Zheng, J. Zhang, N. Li, Y. Chen, B.H. (2015), “Effect of ruthenium
nickel bimetallic composition on the catalytic performance for benzene
hydrogenation to cyclohexane.” Appl. Catal., A. Vol. 499, 124-132.
19