Primary Cell e Newsletter Issue 18 (jun 2016) Cell Applications
· Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E...
Transcript of · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E...
![Page 1: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/1.jpg)
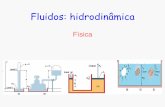
Chapter 22: Introduction to Electroanalytical Chemistry
analyte in solution is part of an electro-chemical cell
advantages:
1. may be specific for specific oxidation state2. relatively cheap instrumentation3. provide activities (effective conc) rather
than concentrations
electrochemistry - interchange of chemical and electrical energy.
oxidation - loss of electronsreduction - gain of electrons
review: balance redox equations
Typical electrochemical cell:
Cu2+ + Zn → Cu + Zn2+
anode - electrode where oxidation occurs cathode - electrode where reduction occurs salt bridge - allows for passage of ions
![Page 2: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/2.jpg)
line notation : Zn / Zn2+( M) // Cu2+( M) / Cu
charge is carried in 3 ways:
1. electrons carry charge in electrodes and in external circuit
2. anions and cations carry charge in solutions and in salt bridge
3. oxidation and reduction at electrode surfaces
galvanic cell - uses a spontaneous redox reaction to generate electricity
electrolytic cell - consume electricity to drive nonspontaneous reaction
cell potential - driving force for electron flow; measured in volts; also called emf or electromotive force
standard reduction potential( E o ) - potential of a half-reaction, written as a reduction, under standard conditions (P = 1 atm, [X] = 1 M), compared to the std hydrogen electrode
2 H+ + 2 e- → H2(g) Eo = 0.0 V
![Page 3: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/3.jpg)
These can be found in tables.
Eocell = Eo
cathode + Eoanode
If Ecell > 0, this is a spontaneous process and the cell is a galvanic cell.
ΔGo = - nFEo n = moles e- changedF = Faraday's cst = 96,485 C/mole e-
1 C = 1 amp x 1 sec
note: ΔG = - RT ln K, so this is one method of determining K
If Ecell < 0, this is a nonspontaneous process and the cell is an electrolytic cell.
dependence of cell potential on concentration is given by the Nernst equation:
E = Eo - RT/nF ln (aproducts / areactants)
At 25o C, this may be simplified to
E = Eo - 0.0591/n log (aproducts / areactants)
![Page 4: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/4.jpg)
so one can calculate Ecell if concentrations are known.
At equilibrium, Ecell = 0.00 V and (aproducts / areactants) = K
so this becomes Eo = 0.0591/n log K,
or log K = nEo / 0.0591.
notes:
liquid junction - interface between two solutions containing different electrolytes or different concentrations of the same electrolyte; will cause a potential difference
all cell voltage measurements measure ΔV; cannot measure absolute V
cell potentials are affected by 1. pH2. precipitates and complexing agents
Types of electroanalytical methods:
![Page 5: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/5.jpg)
Chapter 23: Potentiometry
based on measurement of potential of electro-chemical cells with negligible current drawn
Ecell = Eind + Eref + Ej
Eind = potential of indicator electrodeEref = potential of reference electrodeEj = liquid junction potential
Ideally, Ej = 0.0 V and Eref is constant
Reference electrodes should
1. be easy to assemble2. provide reproducible potential3. remain unchanged in potential with passage
of small amounts of current
Standard Hydrogen Electrode
2 H+ + 2 e- → H2(g) Eo = 0.00 V
![Page 6: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/6.jpg)
Calomel Electrode
Hg2Cl2 + 2 e- → 2 Hg(l) + 2 Cl-
Eo depends on KCl solution conc; for saturated calomel electrode @ 25o C = 0.2444 V
Silver / Silver Chloride Electrode
AgCl(s) + e- → Ag(s) + Cl-
Indicator Electrodes
1. first kind - metal in contact with its ions
Ex: Cu / Cu2+
2. second kind - metal in contact with an anion with which its ion forms a ppt or complex
Ex: Ag / AgCl electrode
3. third kind - metal responsive to other cations
![Page 7: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/7.jpg)
membrane electrodes - many types of ion specific membrane electrodes
glass pH electrode - potential forms across thin glass membrane separating two solutions of different pH. Potential is due to ion exchange processes on membrane surfaces
note: actually measures activity of H+ directly
must standardize pH meter
potentiometric titrations - follow progress of titration by measuring potential
may be used for titrations for which there is no good indicator
nonaqueous titrations
![Page 8: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/8.jpg)
Chapter 24: Coulometry
method is based upon complete oxidation or reduction of an analyte
1. constant-potential coulometry2. constant-current coulometry
both of these measure amt of electricity
3. electrogravimetry - deposit material on electrode and weigh
electrolysis - a nonspontaneous reaction is driven by an application of electricity
overvoltage - amount of potential required above calculated E value to enable reaction to proceed
current-voltage relationships:
1. constant-potential
2. constant-current
![Page 9: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/9.jpg)
Chapter 25: Voltammetry
method based upon current behavior as a function of applied voltage at a polarizable (micro)electrode
This differs from coulometry in that only a small portion of the analyte is oxidized (or reduced), compared to all in coulometry.
Polarography - one of the first techniques; usually utilizes dropping Hg electrode
voltammogram and information obtained:
note: must eliminate dissolved O2 or it will reduce at cathode. This can be done by bubbling an inert gas through the system.
![Page 10: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/10.jpg)
Chapter 26: Introduction to Chromatographic Separations
chromatographic separations - based on physical properties of a substance; the tendency of a substance to be attracted to another substance.
chromatography - differential migration method based upon the rate of migration of a solute in a mobile phase through a stationary phase
mobile phase - provides driving force- usually gas or liquid- force is flow (gravity or pressure)
stationary phase - retarding force- paper, finely divided solid, finely divided solid coated with thin film of viscous liquid- force is adsorption onto solid or
partition between liquid coating of solid and mobile phase
discovered by Russian botanist Tswett
![Page 11: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/11.jpg)
Types of chromatography:
Classification by mobile phase:
1. gas chromatography2. liquid chromatography
Classification by retardation mechanism:
1. adsorption (GSC)2. partition (GLC)
Classification by equipment:
1. column chromatography2. thin-layer chromatography (TLC)3. paper chromatography
chromatogram - plot of amount of analyte detected v. time (or volume)
distribution constant (coefficient):
K = cS / cM cS = conc in stationary phase cM = conc in mobile phase
retention time (tR) - time required for a solute to migrate the length of a column
![Page 12: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/12.jpg)
dead time (tM) - time required for unretained species to migrate length of column
retention factor (k) -
k = (tR - tM) / tM
selectivity factor (α) - (for solutes A and B)
α = KB / KA = kB / kA (note: α > 1)
peak shape - should be Gaussian, but tends to broaden with longer retention times.
column efficiency - usually measured as theoretical plates, which are equilibrium steps in elution.
N = number of platesN = L / H L = column length
H = plate height (HETP)
Observe elution peak. Let tR = x and let y = peak base width.
N = 16 (y/x)2
HETP = L / N
![Page 13: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/13.jpg)
Van Deemter Eq:
HETP = A + B/μ + C μ (μ = mobile phase velocity)
optimal separation when HETP is minimal
resolution(R) - measure of ability of a column to separate two analytes
R = 2( tRB - tRA) / (wA + wB)
notes:
If α = 1, no separation. α depends on nature of liquid phase and on T.
In general, 30o increase in T will halve K, thus doubling rate of component migration.
Lower T = better resolution.
![Page 14: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/14.jpg)
Chapter 27: Gas Chromatography
Mobile phase is gas.
Stationary phase is finely divided solid or finely divided solid coated with nonvolatile liquid contained in a column.
packed column - mobile phase percolates through stationary phase; usually 6 - 10 ft long and 2 - 4 mm id
capillary column - stationary phase lines column wall; usually 150 - 300 μm id and up to 50 m long.
retention volume(VR):
VR = tR F (F = flow rate)
Instrumentation:
carrier gas - must be nonreactive; P constant
usually He or N2
![Page 15: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/15.jpg)
sample injection system - sample must enter column as a vapor in a short period of time in a reproducible manner without upsetting equilibrium of the column
liquid sample - inject into heated metal block with microsyringe through septum. Temperature must be above boiling point for all components, but below decompo-sition point.
gas sample - inject with gas syringe or use vacuum line
solid sample - pyrolize or make volatile derivative
column - support material is usually diatomaceous earth with CaCO3. Particle size is given in mesh (smaller size = larger mesh)
1. must have large surface area2. uniform pore structure3. chemically inert4. thermal stability
![Page 16: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/16.jpg)
- Thousands of specialty phases for liquid coating for GLC; use depends on sample to be separated. Usually use nonpolar phase for nonpolar samples and polar phase for polar samples for maximal retention.
- Sample must be soluble in liquid phase, or no separation will occur. - temperature range for liquid
- liquid must be chemically inert
Temperature - isothermal or ramped; must be reproducible
usually better separation at lower T
Detectors:
1. thermal conductivity detector - based on fact that a hot body will lose heat at a rate dependent upon composition of surrounding gas.
- advantages - cheap, durable, nondestructive
- disadvantages - poor sensitivity
![Page 17: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/17.jpg)
2. flame ionization detector - effluent from column is burned in a small hydrogen flame, producing ions
- advantages - reliable, sensitive- disadvantages - sample destruction; not good for halogens
3. electron capture - effluent is passed through ionization chamber. Organics will interfere with current flow. Must add quenching gas (CH4). Very sensitive for halogenated compounds.
4. specialty detectors - mass spec, UV, IR
Advantages of GC:
1. speed2. resolution3. qualitative analysis (tR)4. quantitative analysis - peak area5. sensitivity
thermal conductivity - 100 ppmflame ionization few ppm
6. simplicity
![Page 18: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/18.jpg)
Chapter 28: High Performance Liquid Chromatography (HPLC)
"most widely used of all analytical separation techniques" since only about 20% of compounds can be analyzed by GC because of 1)volatility or 2) thermal stability.
Early attempts to apply pressure to speed liquid flow through column were unsuccessful due to increase in HETP. This was remedied by smaller particle size.
Mechanisms of separation:
1. partition (L - L)2. adsorption (L - S)3. ion exchange4. size exclusion
Instrumentation:
Pump - considerations1. pressure delivery over broad flow range2. maximum pressure3. compatibility with different solvents4. noise level in detector
![Page 19: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/19.jpg)
reciprocating pump (about 90% of HPLC's)
- small volume chambers with reciprocating pistons to work directly on solvent or use diaphragms and hydraulic fluid.- constant pressure due to out of phase piston relationship
displacement pump (syringe type)
- limited capacity (250 - 500 mL)- pulseless flow- high pressure
pneumatic pump
- mobile phase is in a collapsible container; use gas pressure
injection system:
microsyringe with blunt tipstopped flow systemsample size set by sample loop
![Page 20: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/20.jpg)
column: (packed)
- heavy walled glass or stainless steel- must be smooth walled- generally straight- usually about 5 mm id for analytical
detector: (many types)
bulk property detector - measures change in solvent property, such as refractive index
- fairly insensitive- good temperature control
solute property detector
- UV-vis (usually fixed λ of 254 - 280 nm)- fluorescence- electrochemical
phases:
solvent - must be degassedgradient elution - change composition reverse phase - hydrophobic packing (usually C8 or C18 functional group and polar (usually aqueous) mobile phase
![Page 21: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/21.jpg)
Chapter 11: Atomic Mass Spectrometry
- used for identification and quantification of almost all elements in periodic table
- advantages of technique1. sensitivity2. easy interpretation3. can measure isotope ratios
- disadvantages of technique
1. cost2. instrument stability3. interferences
- steps in analysis
1. atomize sample2. convert to stream of ions3. separate by mass/chg ratio (m/z)4. count number of ions of each type
- note: amu: 1 atom of 12C is exactly 12.00isotopic masses are exactatomic mass is avg of all isotopes
![Page 22: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/22.jpg)
Instrumentation:
Inlet:
1. gas samples2. liquid samples4. solid samples
Ion Source:
1. ICP2. spark3. glow discharge
Mass Analyzer:
1. Quadrupole2. Time-of-flight
Detector:
1. Electron multiplier2. Faraday cup
Readout:
Mass spectrum - plot of mass (x-axis) v. relative abundance (y-axis)
![Page 23: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/23.jpg)
Chapter 20: Molecular Mass Spectroscopy
When a molecule is ionized, it frequently fragments, and the fragmentation pattern is a fingerprint, which can be used for qualitative analysis.
Molecular ion → M+ (parent peak)
Base peak - most abundant ion
Isotope peaks - due to isotopes
Collision product peaks - may be heavier than parent peak (usually M + 1)
Instrumentation:
Inlet systems
batch inlet - vaporize sample externally
direct probe - used for solid or nonvolatile liquids
![Page 24: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/24.jpg)
Sources:
gas phase source - sample is vaporized, then ionized
desorption sources - sample is converted directly to gaseous ions; can be used for nonvolatile or thermally unstable species
hard sources - rupture more bonds than softer sources ex: electron impact v chem. Ionization
Mass analyzers:
magnetic deflection analyzer
KE = mv2 / 2 = constant
M / Z = β2r2e / 2V β = mag. fielde = electron chgV = voltage
![Page 25: · Web viewatm, [X] = 1 M), compared to the std hydrogen electrode 2 H + + 2 e-→ H 2(g) E o = 0.0 V These can be found in tables. E o cell = E o cathode + E o anode If E cell > 0,](https://reader030.fdocument.org/reader030/viewer/2022021504/5ab5a3197f8b9ab7638d00bd/html5/thumbnails/25.jpg)