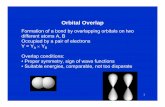
Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet...
Transcript of Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet...
I-2
Contents
• What is VB / Spinfunctions/Bonds • Orbital Optimisation / Brillouin Theorem• Energies and Gradients - Matrix elements• Applications/Illustrations
• Is the BSSE problem solved by using Non-orthogonal Orbitals ?• What is a π system in a bent benzene ?• Resonance and aromaticity• Local Resonance• Do VB structures mean something ?• What about O2
I-3
What Universe are we in:• Time independent• Electronic Structure • Born Oppenheimer• Only scalar relativity• The Answer is 42• The Question ??
H = h(i)i
! +1
rij+ N .R.+ ~
i< j
!
1-electron 2-electron
I-4
Starting from :AtomsBonding : Spin-pairingOrbitals : Non-Orthogonal
(historically) fixedSimplest : 1 structure, more determinantsIn general : Multi-structureExtend : Add Ionic.. structures
VB
MO
Starting from : MoleculesBonding : Sharing OrbitalsOrbitals : Orthogonal
OptimisedIn general : 1 configuration, 1 determinantExtend : Add configurations (CI)
I-5
VB-atoms
H2O O3!g
"
1s22s
22pz
22px2py
1s22s
22px
22pz2py
1s22s
22py
22pz2px
#
$%%
&%%
Ha
2S 1s
a
Hb
2S 1s
b
H2O =O!H !H and
or
! = 1s2h2
2h3
2h1sah4sb
O
h1h2
h3 h4
sa
sb
I-6
SPIN COUPLING
Perfect pairing - two orbitals are coupled to a singlet (bond)
! = N "1" 2# "
1"2( )
1 2
! = "1"2
( )
I-7
1
3
2
213
Two Bonds
! = N "1" 2a
# " 1"2a( ) "
2b" 3# "
2b"3( ) = "
1"2a
( ) "2b"3
( )
! = "1"3
( ) "2 a"2b
( )
A
I-8
VB-2
!(1,2) = c1sa(1)s
b(2)+ s
a(2)s
b(1)( )
Covalent
! "#### $####
+c2sa(1)s
a(2)( )
ionisch
! "# $#+ c
3sb(1)s
b(2)( )
ionisch
! "# $#
H2 vergeet spin
Covalent ionisch ionisch
I-9
H2 VB possibilities!singlet =
1
21sA1sB +1sB1sA( ) "# $ #"( ) ms = 0, s = 0 (singlet %&)
! triplet =
1
21sA1sB $1sB1sA( ) "# + #"( ) ms = 0, s = 1 (triplet %%)
1
21sA1sB $1sB1sA( ) ""( ) ms = 1, s = 1 (triplet %%)
1
21sA1sB $1sB1sA( ) ##( ) ms = $1, s = 1 (triplet %%)
'
(
))))))
Kunt E =! H !
! ! berekenen; Grondtoestand is natuurlijk singlet
De Hamiltoniaan bevat (bij ons) enkel ruimte afhankelijketermen. Dus wordt de energie door het ruimte deel bepaald.
Volgorde elektronen - 1..2 1..2
I-10
VB energy H2 - 3!singlet :
1
21sA1sB +1sB1sA( ) (singlet "#) E $ E1 + J + K
! triplet :
1
21sA1sB %1sB1sA( ) (triplet "")
1
21sA1sB %1sB1sA( ) (triplet "")
1
21sA1sB %1sB1sA( ) (triplet "")
&
'
((((((
E $ E1 + J % K
J en K : Bevoordelen triplet (denk He* / Regel van Hund)VB attractief door 1-elektron term (niet orthogonaal)But J,K,E are different
gives bonding
I-11
Can we use orthogonal Orbitals in VB ??
H2(STO6G) ! = 1
2ab + ba [ ]
VB: Heitler-London : 0.70 Å 120.6 Kcal/molVB: Coulson-Fischer: (full CI) 0.73 Å 128.0 Kcal/molHartree-Fock : 0.71 Å 115.6 Kcal/molOrthogonal VB : >5.9 Å 0 Kcal/molExperimental (WWW): 0.74 Å 104 Kcal/mol
fixed orbitals optimised orbitals
NO
I-12
Atomic Population Analysis (cf. McWeeny (1953))
H2 (STO6G/0.70 Å)
Orb-overlap qa qab EnergyVB: H-L 0.69 0.68 0.32 -1.134187VB: C-F 0.81 0.62 0.38 -1.144979HF : 0.0 0.60 0.40 -1.126100Orthog VB : 0.0 1.89 -0.89 -0.463516
VB:C-F (+p) 0.81 0.61 0.39 -1.148056
I-13
VB-hierarchy
! = C
i"
i# L Wavefunction
! = s
d"d# L Structure
! = "1"2..."
nL Determinant
! = c
i"i# L Orbital
(H ! ES)C = 0 or fixed
Spin-projection
Optimised or fixed basis-function
I-14
Spin-Functions(1)Rumer functions : Different orbitals spin-paired
Benzene (6-31G)
!1
= N "1" 2# "
1"2( ) "
3" 4# "
3"4( ) "
5" 6# "
5"6( )
-230.6614 -230.6614-230.6326-230.6326 -230.6326
0.44 0.44-0.07-0.07 -0.07CE = -230.6938
Spin-functions non-orthogonal!1!4 spin
= 0.25 !1!3 spin
= 0.5
!1!4
= 0.66 !1!3
= 0.82!1!2
= 0.52
C5
C4
C3
C2
C1
C6
C5
C4
C3
C2
C1
C6
C5
C4
C3
C2
C1
C6
C5
C4
C3
C2
C1
C6
C5
C4
C3
C2
C1
C6
I-15
Spin-Functions(2)
Branching Diagrams : Orbitals in standard orderDifferent spin-functions
1 2
Spin-functions orthogonal;Obtain by e.g Schmidt orthogonalising Rumer functions
5 4
I-16
# Atomic states Configuration spin Coefficient1 O(3P) H(2S) 1s22s2y2xzh -0.2352 O(1D) H(2S) 1s22s2y2xyz 0.1913 O+(2D0) H-(1S) 1s22s2(z2-y2)xh2 0.0534 O+(2P0) H-(1S) 1s22s2(z2+y2)xh2 -0.0285 O-(2P0)H+ 1s22s2z2y2x 0.327
Spin-Functions(3)
Branching Diagrams are often considered less informativeOH (2Π) (JHvL, G.G.Balint-Kurti (1980) DZP, Atomic Opt
!
• Oxygen 1D mixes in• O- ionic structure important due to atomic orbitals• O(z) => hybrid on bond formation
• Spin-function may be transformed from one basis to another
I-17
VB=MCSCF
! = N c1"3" 3# c
2" 4"4( ) =
with
"1
=c1"3
+ c2"4( )c1
+ c2( )
"2
=c1"3# c
2"4( )c1
+ c2
( )
$
%
& &
'
& &
(
)
& &
*
& &
= N "1" 2# "
1"2( ) = "
1"2( )
"1"2
=c1# c
2
c1
+ c2
"3"4
= 0
I-18
!
1!
2( ) !3!
4( ) = 2 " MCSCF( )* 2 " MCSCF( )
Only if (φ1φ2) orthogonal to (φ3φ4) :Strong Orthogonality
GVB : W.A. Goddard III et al (1972 - ..)Original version: generalPopular version : GVB-PP
strong orthogonalityperfect pairinguses Fock-operators (N4)size extensive
1999 : GVB-RP
I-19
Spincoupled / Spincoupled VBJ. Gerratt†, M. Raimondi, D.L. Cooper - “The gang of Three”
Spincoupled : 1 configuration all spincouplings, optimised
! = cSk N!( )
12
A "1"
2"
3..." N#S ,M ;k
N{ }k
fSN
$
Fi
eff! i = "i! i
Each ψi satisfies her own
“stack” of virtual orbitals (ψi1,ψi
2,….)
Generate excited structures by applying excitations :
Ci!i1 orCi!i 2 ,... (singles),Ci!i1, j!ij1 (double)
Spincoupled-VB
This function can be obtained by restricting a CASSCF function : CASVB
I-20
Orbital models
Fixed (VB)Optimised
AtomicPartial DelocalDelocalBreathing
F F
covalent ionic Bionic A
(overlaps to 99 %)
I-21
+G. Balint-Kurti, G. Gallup, R. McWeeny, J. van Lenthe,M. Raimondi, Y. Mo, W.Wu, R. Harcourt, D. Cooper, J.Gerratt,R. Braam, , P-A Malmquist, R. Havenith,S. Shaik, P. Hiberty, S. Humbel, P. Karadov + .....and a lot of Physics (superconductivity, crystals) e.g.Masafumi Tamura, Akiko Nakao, Reizo Kato,“Frustration-induced valence - bond ordering in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. “
XMVB, CASVB, TURTLE, VB2000, CRUNCH,...
I-22
(Core)-hole states / Broken symmetry
eA AB B
Hit 1s
! =!A!
B
+
! =!A
1
2+
!B
1
2+
! =!A!
B
+
"!B!
A
+
!
A!
B
+
!A
+
!B
> 0.9 Ria Broer - GNOME
Glyoxaal+HC
O
CH
O+
Paul Ruttink
He2+
I-23
NH (3Σ-) dimerG.Dhont, G. Groenenboom,A. vd Avoird
VB allows states to remain “themselves” as interaction (and overlap) kicks in
I-24
Some wise (edited) remarks by J.N. Murrell, S.F.A. Kettle and J.M. Tedder
The Chemical Bond(Wiley, 1985)
Valence bond theory relies on chemical intuition for finding suitablevalence structures. In some respect, this may seem an advantage in that chemicalexperience can be directly introduced into a quantum-chemical calculation. Inanother respect, however, it is a weakness because it assumes some knowledge ofthe answer at the start. If chemical intuition is wrong, the VB calculation is wrong.The most valuable calculations are often those whose results refute intuition.In contrast, molecular orbital theory does not make any assumption about the wayorbitals are paired in a molecule.
Probably the most misunderstood concept of the theory is “resonance energy”.From resonance theory we deduce that a resonance hybrid will have a lower energythan any single canonical form. The theory tells us nothing about the absolutestability of one species relative to another.
![Page 1: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/1.jpg)
![Page 2: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/2.jpg)
![Page 3: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/3.jpg)
![Page 4: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/4.jpg)
![Page 5: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/5.jpg)
![Page 6: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/6.jpg)
![Page 7: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/7.jpg)
![Page 8: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/8.jpg)
![Page 9: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/9.jpg)
![Page 10: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/10.jpg)
![Page 11: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/11.jpg)
![Page 12: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/12.jpg)
![Page 13: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/13.jpg)
![Page 14: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/14.jpg)
![Page 15: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/15.jpg)
![Page 16: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/16.jpg)
![Page 17: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/17.jpg)
![Page 18: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/18.jpg)
![Page 19: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/19.jpg)
![Page 20: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/20.jpg)
![Page 21: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/21.jpg)
![Page 22: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/22.jpg)
![Page 23: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/23.jpg)
![Page 24: Valence Bond - ACMM · Valence Bond Joop van Lenthe ... in a new quantum triangular antiferromagnet based on [Pd(dmit)2]. ... I-Begin.ppt Author: Joop van Lenthe](https://reader042.fdocument.org/reader042/viewer/2022022009/5af4b0777f8b9a4d4d8e02b1/html5/thumbnails/24.jpg)