S1. Free energy of hydrogen atom, proton and electron10.1007/s00894-014-2509...Free energy of...
Transcript of S1. Free energy of hydrogen atom, proton and electron10.1007/s00894-014-2509...Free energy of...

1
S1. Free energy of hydrogen atom, proton and electron
The free energies of particles like hydrogen atom, proton and electron in different medium, which are used
in this work, are given in the following Table 1.s. We have collected them from Jan Rimarck’s work done in
2010 [41].
Table 1.s. Solvation free energies ΔG°solv of hydrogen atom H●, proton H+ and electron e- in kJ/mol
Solvent ε ΔG°solv(H●) ΔG°solv(H+) ΔG°solv(e-)Cyclohexane 2.02 -20 -772.0 0.2Benzene 2.28 -20 -968.4 -13.0Dichloromethane 8.93 -20 -1423.5 -338.0Methanol 32.7 -20 -1012.1 -84.0Water 78.4 27.7 -995.9 -99.3
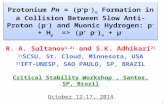
S2. Relaxed potential energy surface of vibrational well
The vibrational potential wells in Figure 1.s. are obtained by scanning O-H bond at B3LYP/6-31G level
of theory. In this we have calculated the potential energy of the O-H bond for 30 points with a step of
0.075Ǻ starting from 0.675 Ǻ for all the molecules studied. It has been done for either site (position 3 and
4). Then we have set the molecular geometry of the lowest point of the well and the highest point (30th
point) of all the potential wells to make optimization at B3LYP/6-31+G(d,p) level of theory. This has
allowed us to calculate the depth of each well which is the energy range of both geometries found. We have
done calculations only in benzene, methanol and water to find energy range which are collected in Table 1.s.
The data displayed in Table 2.s. show that the well in site 4 is deeper than the one in site 3 for all the
molecular trio (CAPE, MBC and BC). These results agree with the BDE parameter in our previous work [7]
which states that, it is easy to lift hydrogen atom from site 3 rather than the one in site 4 in vacuum as in
solvents. It is noticeable that, the higher the dielectric constant the lower the depth of vibrational potential
well. Therefore, solvents contribute to fill in well, in the other words; they contribute to diminish the depth
of vibrational potential well. The depths of vibrational potential of well of molecules are ordered as follow:
depth(CAPE3) ~ depth(MBC3) < depth(BC3) < depth(P4HC) < depth(P3HC). This order is in good
agreement with the one obtained by BDE3 [7]. In solvents, CAPE loses its AA power, because it digs
forward its well compared to BC and MBC molecules. Thus, the depth can be used to describe antioxidant
activity of phenolic compounds.
S3. O-H bond vibration
The frequency is defined as the speed of vibration. From our calculation, we have collected the
vibrational frequency data of O-H bond of all molecules studied in both position (3 and 4) displayed in our
previous work [7]. The value of the vibrational frequency O-H bond of CAPE is 3842.9cm-1 in site 3 and
3774.5cm-1 in site 4. However, the IR spectrum done by Son et al [5] showed a peak for phenolic -OH at
3490cm-1. This value is lower than that found by calculation; because Son has found it by X-ray diffraction
on a single crystal of CAPE. Since in that crystal, each CAPE sees the other ones, then it is normal that the

2
frequency value of O-H bond reduces; this is because all O-H bonds are choked by the surrounding
molecules.
Table 2.s. vibrational potential depth of O-H bond of CAPEn (n=3,4) and its derivative in conformer BSolvents Vacuum Benzene Methanol WaterCAPE3 0.1880 0.1880 0.1872 0.1870CAPE4 0.1988 0.1953 0.1910 0.1908MBC3 0.1880 0.1880 0.1866 0.1869MBC4 0.1984 0.1949 0.1908 0.1904BC3 0.1888 0.1887 0.1870 0.1868BC4 0.1988 0.1951 0.1905 0.1902P3HC 0.2027 0.2022 0.2008 0.2007P4HC 0.1953 0.1949 0.1932 0.1930
0,5 1,0 1,5 2,0 2,5 3,0
-958,10
-958,05
-958,00
-957,95
-957,90
-957,85
-957,80
-957,75
-957,70
0.18
80au
B-CAPE3
En
erg
y in
a.u
.
O3-H
3 bond distance
0,5 1,0 1,5 2,0 2,5 3,0
-958,10
-958,05
-958,00
-957,95
-957,90
-957,85
-957,80
-957,75
-957,70
0.19
88au
B-CAPE4
En
ergy
in a
.u.
O4-H
4 bond distance
0,5 1,0 1,5 2,0 2,5 3,0-843,80
-843,75
-843,70
-843,65
-843,60
-843,55
-843,50
-843,45
-843,40
0.18
78au
B-MBC3
En
erg
y in
a.u
.
O3-H
3 bond distance
0,5 1,0 1,5 2,0 2,5 3,0-843,80
-843,75
-843,70
-843,65
-843,60
-843,55
-843,50
-843,45
-843,40
0.19
85au
B-MBC4
Ene
rgy
in a
.u.
O4-H4 bond distance
0,5 1,0 1,5 2,0 2,5 3,0-918,80
-918,75
-918,70
-918,65
-918,60
-918,55
-918,50
-918,45
-918,40
0.18
88au
B-BC3
En
ergy
in a
.u.
O3-H
3 bond distance
0,5 1,0 1,5 2,0 2,5 3,0-918,80
-918,75
-918,70
-918,65
-918,60
-918,55
-918,50
-918,45
-918,40
0.19
88au
B-BC4
En
erg
y in
a.u
.
O4-H4 bond distance

3
0,5 1,0 1,5 2,0 2,5 3,0
-882,90
-882,85
-882,80
-882,75
-882,70
-882,65
-882,60
-882,55
-882,50
0.20
27au
B-P3HC
En
ergy
in a
.u.
O3-H
3 bond distance
0,5 1,0 1,5 2,0 2,5 3,0
-882,90
-882,85
-882,80
-882,75
-882,70
-882,65
-882,60
-882,55
-882,50
0.19
53au
B-P4HC
Ene
rgy
in a
.u.
O4-H
4 bond distance
Fig.1.s. RPES obtained from hydrogen displacement of O-H bonding
1,01,1
1,21,3
1,41,5
1,61,7
1,8
-994,630
-994,625
-994,620
-994,615
-994,610
-994,605
-994,600
-994,595
106108
110112
114116
118120
122
En
ergy
in a
.u.
C 3-O
3-H 3
(°)
R(O3 -H
3 ) in Angström
B-MBC + HO.2
1,01,1
1,21,3
1,41,5
1,61,7
1,8
-1069,635
-1069,630
-1069,625
-1069,620
-1069,615
-1069,610
-1069,605
-1069,600
106108
110112
114116
118120
122
En
ergy
in a
.u.
C 3-O 3
-H 3 (°
)R(O
3 -H3 ) in Angström
B-BC + HO.2
1,0 1,1 1,2 1,3 1,41,5
1,61,7
1,81,9
-1033,75
-1033,74
-1033,73
-1033,72
-1033,71
-1033,70
-1033,69
-1033,68
104106
108110
112114
116118
120
Ene
rgy
(au
)
C 3-O
3-H
3 (°
)
R(O3-H
3) in Angström
B-P3HC + HO.2
1,0 1,1 1,2 1,3 1,4 1,5 1,6 1,7 1,8 1,9 2,0
-1034,06
-1034,05
-1034,04
-1034,03
-1034,02
-1034,01
-1034,00
104106
108110
112114
116118
120
Ene
rgy
in a
.u.
C 4-O 4
-H 4 (°
)
R(O4-H
4) in Angström
B-P4HC + HO.2
Fig.2.s. 3D-RPES of the reaction of B-MBC with HO2●, B-BC with HO2
●, B-P3HC with HO2●
and B-P4HC with HO2●

4
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
200
300
400
500
600
700
800 B-CAPE B-MBC B-BC B-P3HC
Fre
e en
erg
y of
rea
ctio
n
1 3 in
kJ/
mo
l
solvents
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
100
200
300
400
500
600
700
800 B-CAPE B-MBC B-BC B-P4HC
Fre
e e
ner
gy o
f re
act
ion 1 4
in k
J/m
ol
solvents
Fig.6.s. Free energy of reaction Γ13 (right) and reaction Γ1
4 (left) of B-CAPE, B-P4HC, P3HC, B-MBC and B-BC each other in the presence of radical HO2
● in different media
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
0
100
200
300
400
500
600B-MBC reaction A
3 (CPCET)
reaction B1 (ET-PT)
reaction 2
3 (SPLET)
Fre
e en
ergy
in k
J/m
ol
solvents
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
-200
-150
-100
-50
0
50
B-MBC
reaction A3 (CPCET)
reaction 1
3 (PT-ET)F
ree
en
erg
y in
kJ/
mo
l
solvents
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
0
100
200
300
400
500
600B-BC reaction A
3 (CPCET)
reaction B1 (ET-PT)
reaction 2
3 (SPLET)
Fre
e e
ne
rgy
in k
J/m
ol
solvents
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
-200
-150
-100
-50
0
50
B-BC
reaction A3 (CPCET)
reaction 1
3 (PT-ET)
Fre
e e
ne
rgy
in k
J/m
ol
solvents
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
0
100
200
300
400
500
600 B-P3HC reaction A3 (CPCET)
reaction B1 (ET-PT)
reaction 2
3 (SPLET)
Fre
e en
erg
y in
kJ/
mol
solvents
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
-150
-100
-50
0
50
100
B-P3HC
reaction A3 (CPCET)
reaction 1
3 (PT-ET)
Fre
e e
nerg
y in
kJ/
mo
l
solvents

5
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
0
100
200
300
400
500
600B-P4HC reaction A
4 (CPCET)
reaction B1 (ET-PT)
reaction 2
4 (SPLET)
Fre
e en
erg
y in
kJ/
mol
solvents
Vacuum
CyclohexaneBenzene
DichloromethaneMethanol
Water
-200
-150
-100
-50
0
50
100
B-P4HC
reaction A3 (CPCET)
reaction 1
3 (PT-ET)
Fre
e e
nerg
y in
kJ/
mo
l
solvents
Fig.9.s. Free energy of reaction An, B1
n and Γ1 (n=3,4) of B-P4HC, P3HC, B-MBC and B-BC in the presence of radical HO2
● (right) and radical O2●- (left) in different media.
S4. The equilibrium geometry coordinates of transition state
i. The transition state coordinates of reaction involving BCAPE3 + HO2● in vacuum
C 5.01657200 -2.12048300 0.83866600C 3.63349900 -2.19959600 0.79510500C 2.81257600 -1.06921000 0.49173900C 3.42052400 0.15419800 0.21944500C 4.82705200 0.27600500 0.25916900C 5.62451900 -0.89267000 0.57967800H 2.84874900 1.04502400 -0.01684600O 5.50209000 1.36059100 0.02737900O 6.94915700 -0.73880500 0.61605800H 7.11178400 0.20458000 0.40665200H 5.63111100 -2.98226600 1.07328800H 3.14939500 -3.15116500 0.99312400C 1.36680100 -1.26749800 0.47629400H 1.01508000 -2.27686000 0.68246300C 0.41696500 -0.34254300 0.24087000H 0.63913200 0.69756800 0.02742400C -1.00976000 -0.73066600 0.27276700O -1.43668500 -1.84874100 0.49410100O -1.80105400 0.33831900 0.02049800C -3.22211100 0.08488500 0.02966300H -3.46005100 -0.66875900 -0.72772900H -3.50990400 -0.32548800 1.00264700C -3.92429300 1.41136400 -0.25359300H -3.57773000 1.79362100 -1.21988400H -3.61940600 2.13859700 0.50685400C -5.43025000 1.25199300 -0.25787300C -6.11506900 0.93807000 -1.43919400H -5.55933800 0.84217200 -2.36901400C -7.49825700 0.75550400 -1.43803600H -8.01191100 0.51701600 -2.36496700C -8.22020200 0.88401700 -0.25058700H -9.29732300 0.74531300 -0.24837300C -7.55032200 1.19756000 0.93304500H -8.10483700 1.30484200 1.86090800C -6.16717300 1.37924500 0.92708100H -5.65220000 1.62896800 1.85174800H 4.63667000 2.46083300 0.05002600O 3.70456300 3.46785500 0.07625700O 3.72304500 3.83626500 -1.30417000H 3.43065900 4.76023700 -1.24025600
ii. The transition state coordinates of reaction involving BCAPE3 + HO2● in benzene
C -5.36825600 -2.11534700 0.16162500C -3.99913300 -2.29954900 0.16285300C -3.06788400 -1.20942200 0.01802000C -3.55245700 0.07830800 -0.13229600C -4.95322100 0.31566900 -0.13626200C -5.86444500 -0.81720300 0.01205400H -2.91036000 0.94454000 -0.25728700

6
O -5.50794000 1.46680000 -0.26259000O -7.16550600 -0.56296300 0.00003300H -7.26350900 0.40577600 -0.11715300H -6.05681000 -2.94507800 0.27107800H -3.60000800 -3.30242700 0.27773400C -1.64406600 -1.53652400 0.03805600H -1.39256400 -2.59180700 0.11978500C -0.61641400 -0.66972600 -0.02802700H -0.74908800 0.40425600 -0.10212900C 0.77630400 -1.17156300 0.00705700O 1.10485800 -2.34298000 0.09020800O 1.65151500 -0.14898100 -0.06406300C 3.05502100 -0.50119500 -0.03078000H 3.26063700 -1.06372500 0.88477900H 3.28200100 -1.15022100 -0.88234100C 3.85114700 0.80024500 -0.08498100H 3.55211600 1.42975900 0.75983500H 3.58633900 1.33834600 -1.00133300C 5.34177900 0.53655100 -0.04148700C 6.01772300 0.45128500 1.18354100H 5.46794500 0.60622100 2.10867500C 7.38588100 0.17847400 1.22879200H 7.89325000 0.12126000 2.18749800C 8.10106400 -0.01516400 0.04510500H 9.16626600 -0.22392800 0.07831400C 7.43922000 0.06678300 -1.18177500H 7.98826000 -0.07763000 -2.10779200C 6.07084100 0.33980200 -1.22245500H 5.56291500 0.40777400 -2.18138800H -4.51615800 2.44013100 -0.43286600O -3.46942400 3.31345700 -0.59178200O -3.29204800 3.77550000 0.78722700H -3.68151100 4.66154400 0.72809000
iii. The transition state coordinates of reaction involving BCAPE3 + HO2● in water
C -3.10431800 -1.16893800 0.01035500C -3.57676100 0.13438900 -0.07862500C -4.96923000 0.38795100 -0.10657900C -5.88685200 -0.74068400 -0.05423200C -5.41552200 -2.05415200 0.03689000C -4.05245400 -2.26343300 0.06741200H -2.90181200 0.98894000 -0.11088200O -5.52657700 1.55010400 -0.18213800H -4.56089600 2.53456600 -0.35925200O -7.18741300 -0.49331100 -0.08092400H -7.32207300 0.47838200 -0.14663000H -6.10972500 -2.88569000 0.08128800H -3.66079800 -3.27621900 0.14197300C -1.68704400 -1.52380800 0.06607300H -1.47825200 -2.58553400 0.22785400C -0.63045600 -0.69508700 -0.07094700H -0.72790900 0.37956100 -0.24254000C 0.75782800 -1.21200100 0.06085500O 1.06498000 -2.38977700 0.24197700O 1.65109200 -0.22032800 -0.05409400C 3.06263800 -0.56448100 0.17662900H 3.18971600 -0.84677500 1.26985800H 3.37805400 -1.45082800 -0.46674300C 3.85223900 0.73161500 -0.20051300H 3.57847100 1.55951300 0.50857600H 3.56517600 1.04792500 -1.23832600C 5.34538800 0.47031500 -0.13421700C 5.98780100 0.37991500 1.11951300H 5.40075700 0.48241000 2.02923700C 7.37086100 0.15535700 1.19771800H 7.85200400 0.08334500 2.16522900C 8.12500100 0.02657200 0.02764300H 9.20260800 -0.13579900 0.08525800C 7.49618900 0.13475400 -1.21825700H 8.08262900 0.04202400 -2.13869400C 6.12281000 0.34942400 -1.30134700H 5.64127000 0.44157100 -2.26897700O -3.52017900 3.40859400 -0.55053400O -3.02507100 3.58005800 0.83329300H -3.51257600 4.38364000 1.11188600
iv. The transition state coordinates of reaction involving BCAPE4 + HO2● in vacuum

7
C 4.71937200 2.00610800 0.00368400C 3.35469900 2.19601700 0.00149700C 2.44877100 1.09937300 -0.00114400C 2.98298200 -0.20998200 -0.00243000C 4.34919200 -0.41822200 -0.00062400C 5.27264700 0.69411800 0.00384400H 2.31761500 -1.06943700 -0.00505300O 4.93946800 -1.64005100 -0.00272300H 4.27795700 -2.34528100 -0.00570600O 6.54990900 0.52326200 0.00829800H 5.41272800 2.84007100 0.00591000H 2.95190900 3.20471500 0.00184200C 1.02683600 1.37794000 -0.00258700H 0.74655900 2.43001800 -0.00217800C 0.00641700 0.48756700 -0.00425200H 0.15495200 -0.58709900 -0.00479300C -1.39142600 0.95865800 -0.00517300O -1.75257000 2.12358900 -0.00488800O -2.25359200 -0.08914500 -0.00650100C -3.66207100 0.24167500 -0.00672300H -3.88613500 0.84714900 0.87690300H -3.88598800 0.84568700 -0.89140400C -4.44117900 -1.07261400 -0.00575800H -4.14817100 -1.65614600 0.87424800H -4.15407800 -1.65429400 -0.88893200C -5.93724200 -0.83228900 -0.00060800C -6.63968600 -0.70378000 1.20669700H -6.10701100 -0.80727800 2.14929900C -8.01403000 -0.45237200 1.21421600H -8.54039800 -0.36001500 2.16002200C -8.71013200 -0.32545600 0.00912000H -9.77900200 -0.13284000 0.01287500C -8.02259600 -0.45280000 -1.20082700H -8.55564000 -0.36075400 -2.14291600C -6.64822300 -0.70425200 -1.20294700H -6.12222200 -0.80815000 -2.14924300H 7.18278200 -0.74774800 0.00342400O 7.98546400 -1.99554300 -0.00144400O 9.30017200 -1.50328100 0.00388800H 9.81735000 -2.32735600 -0.00324900
v. The transition state coordinates of reaction involving BMBC3 + HO2● in vacuum
C 4.41770000 -2.07062600 0.62013800C 3.06218300 -2.34795700 0.53182200C 2.08667300 -1.32597400 0.31949600C 2.51154300 -0.00456600 0.18961400C 3.88217800 0.31630400 0.27708400C 4.84025600 -0.74734100 0.50043600H 1.81710700 0.81231100 0.02488000O 4.38994000 1.50624500 0.17111300O 6.12786700 -0.40514000 0.58474200H 6.14925100 0.56795700 0.47166400H 5.15085800 -2.85231200 0.78416000H 2.72283200 -3.37549300 0.62142300C 0.68732700 -1.73174200 0.24225500H 0.48746200 -2.79690600 0.34471200C -0.38649500 -0.93944600 0.06173100H -0.31961200 0.13750000 -0.05026900C -1.74003100 -1.53435700 0.01263300O -1.99649000 -2.72069000 0.11644200O -2.67661600 -0.57568400 -0.16232400C -4.04877500 -1.05058500 -0.23500800H -4.10391500 -1.79013300 -1.04294000H -4.28902000 -1.57228600 0.69379200C -4.92224300 0.13054300 -0.50753900H -4.64829900 0.69214600 -1.39959200C -5.98672800 0.53950700 0.19923100C -6.50081100 -0.12107900 1.45410600H -5.87327000 -0.94158300 1.80512200H -7.51168000 -0.51773500 1.29416100H -6.58095700 0.61378500 2.26478600C -6.78506500 1.74079600 -0.24525600H -6.38108000 2.18787100 -1.15714200H -7.83225500 1.46972000 -0.43247400H -6.79879500 2.51062300 0.53702700H 3.44481100 2.53782000 0.22191000O 2.43671400 3.49114800 0.30186100O 2.52520300 4.04288000 -1.00707700H 2.08718500 4.89856800 -0.86605300

8
vi. The transition state coordinates of reaction involving BMBC3 + HO2● in benzene
C 4.38780700 -2.09521300 0.37363400C 3.02630500 -2.33018100 0.34858500C 2.05465700 -1.26977000 0.26389200C 2.49352900 0.04213500 0.20908300C 3.88423100 0.32954600 0.22736800C 4.83679600 -0.77307100 0.31720100H 1.82055200 0.89211400 0.16443700O 4.38994000 1.50624500 0.17111300O 6.12739700 -0.46910700 0.33945300H 6.18823500 0.50855000 0.29220900H 5.10574600 -2.90449000 0.43890900H 2.66519200 -3.35283900 0.39360700C 0.64469400 -1.65241100 0.24194500H 0.43117500 -2.71268900 0.35926200C -0.41137200 -0.83347400 0.08250400H -0.31604600 0.23829800 -0.05560200C -1.78466000 -1.38970400 0.08090800O -2.06770200 -2.56825700 0.22246800O -2.69295300 -0.41337800 -0.09956500C -4.08891200 -0.83897600 -0.13795400H -4.17061500 -1.63538900 -0.88576700H -4.34565700 -1.26958600 0.83153300C -4.91327400 0.35073400 -0.50616700H -4.66045000 0.79115200 -1.46965400C -5.91538800 0.89816000 0.20009900C -6.39467400 0.41658800 1.54656800H -5.80251800 -0.40319500 1.95583900H -7.43736800 0.08036300 1.48346200H -6.37837700 1.24105300 2.26994100C -6.67178400 2.08550900 -0.34412100H -6.29250500 2.40148900 -1.31917300H -7.73963800 1.85425000 -0.44876900H -6.60461000 2.93713500 0.34476900H 3.44481100 2.53782000 0.22191000O 2.43671400 3.49114800 0.30186100O 2.29872600 3.85501300 -1.10837700H 2.70679300 4.73469500 -1.10457500
vii. The transition state coordinates of reaction involving BMBC3 + HO2● in water
C 4.39179800 -2.07458800 0.17932300C 3.04314400 -2.32473800 0.04227900C 2.05702100 -1.27219200 -0.03382500C 2.46901300 0.04452500 0.04493500C 3.84595100 0.34938600 0.19597500C 4.81491600 -0.74620000 0.25786300H 1.78019100 0.88319800 -0.01203200O 4.33460100 1.54370400 0.28117200O 6.09372400 -0.42909300 0.39423500H 6.15869000 0.54526300 0.43488600H 5.11916200 -2.87843500 0.22677500H 2.70055700 -3.36080500 -0.02435000C 0.66274200 -1.68068700 -0.18197800H 0.48535600 -2.75047100 -0.25330400C -0.42459700 -0.87405000 -0.22901400H -0.35914200 0.20582900 -0.15418300C -1.76909900 -1.45999500 -0.37432800O -2.02226900 -2.65407300 -0.50503500O -2.71542300 -0.50408900 -0.34582900C -4.10185400 -0.94400700 -0.49659700H -4.15499400 -1.53509100 -1.41678700H -4.34173900 -1.61005300 0.33774900C -4.96774700 0.26798100 -0.57535800H -4.75217600 0.91559300 -1.42426900C -5.96517500 0.61434900 0.27155700C -6.39820600 -0.17502900 1.47535900H -5.77694200 -1.04418800 1.68146900H -7.43682700 -0.51747800 1.35362700H -6.39164900 0.46859400 2.37337000C -6.76405800 1.86615000 0.02011600H -6.42577400 2.40720600 -0.86939000H -7.83053900 1.63012000 -0.10542000H -6.70233700 2.54429100 0.87993000H 3.37704200 2.54553200 0.28579700O 2.33645300 3.49772800 0.30539100

9
O 2.71922000 4.40012400 -0.92837600H 3.21759200 5.18106100 -0.43417600
viii. The transition state coordinates of reaction involving BMBC4 + HO2● in vacuum
C -3.66132300 -2.00234200 0.19991600C -2.30524700 -2.23901500 0.13824200C -1.36833600 -1.17834000 -0.00513200C -1.86214100 0.14429400 -0.08444900C -3.21911700 0.39911600 -0.02484800C -4.17405000 -0.67639000 0.12117000H -1.17266500 0.97730400 -0.19452600O -3.77148400 1.63652300 -0.09539100H -3.09058400 2.31576700 -0.19523800O -5.44355900 -0.46113300 0.17827100H -4.37807800 -2.80900500 0.30870100H -1.93335600 -3.25770100 0.19950900C 0.04239100 -1.50474600 -0.06448500H 0.29137100 -2.56248600 0.00299000C 1.08562400 -0.65138600 -0.19407500H 0.96805600 0.42448000 -0.26899900C 2.46862300 -1.16523000 -0.23896500O 2.79478900 -2.33956600 -0.17157400O 3.35495900 -0.14895800 -0.36489300C 4.76018100 -0.53274900 -0.43122200H 5.01577800 -1.06931500 0.48410800H 4.87154000 -1.23351400 -1.26634000C 5.56256000 0.70954700 -0.64582300H 5.32564200 1.24682600 -1.56355800C 6.53197600 1.20130500 0.14546000C 6.98498400 0.58945300 1.44895500H 6.39631800 -0.27676900 1.75536000H 6.93395500 1.33418100 2.25333100H 8.03477000 0.27546200 1.38076300C 7.27643200 2.45620500 -0.24471300H 6.91813600 2.86786200 -1.19225100H 7.16890900 3.22822900 0.52842500H 8.35208200 2.25846600 -0.34197700H -6.03064800 0.82930000 0.08538600O -6.78964000 2.10365500 0.00438300O -8.11990400 1.66551600 0.10748400H -8.60784700 2.50428000 0.03788500
ix. The transition state coordinates of reaction involving BBC3 + HO2● in vacuum
C 4.99429300 -1.72519000 0.91808100C 3.67678100 -2.15716500 0.93149800C 2.58463300 -1.27707700 0.66501900C 2.85080900 0.06262600 0.38186200C 4.17729700 0.53838500 0.36445800C 5.25806100 -0.38437500 0.64145700H 2.05999400 0.77623300 0.17693300O 4.54286300 1.76123400 0.11485700O 6.50166100 0.10236000 0.62323700H 6.40872000 1.05478500 0.41387600H 5.81824700 -2.39948300 1.12283800H 3.46122200 -3.19992500 1.14404800C 1.23868900 -1.83881300 0.69441500H 1.16188600 -2.89424100 0.94983300C 0.07953700 -1.20239400 0.43838500H 0.02306400 -0.15333000 0.16847500C -1.19796500 -1.94223300 0.51671600O -1.32154900 -3.11928300 0.80392000O -2.23911900 -1.12879100 0.22106500C -3.54758400 -1.75660600 0.26269300H -3.53731100 -2.62008500 -0.40917100H -3.72568400 -2.12857000 1.27580700C -4.57221700 -0.73493200 -0.14725300C -4.78918300 -0.45349400 -1.50216600H -4.21734600 -0.98710500 -2.25692300C -5.72830200 0.50206100 -1.88664800H -5.88873300 0.71005200 -2.94048000C -6.46554500 1.18642400 -0.91761900H -7.20088600 1.92786200 -1.21624200C -6.25791100 0.91216500 0.43461900H -6.83008300 1.43985800 1.19202100C -5.31456200 -0.04284700 0.81577900H -5.15327100 -0.25501000 1.86955200

10
H 3.48071100 2.67260000 0.15008500O 2.35983800 3.51564900 0.22439600O 2.29567600 3.95118100 -1.12482500H 1.75657000 4.75384600 -1.02658200
x. The transition state coordinates of reaction involving BBC3 + HO2● in benzene
C -4.95341400 -1.77752200 0.12853600C -3.62942400 -2.17469700 0.11742500C -2.54212100 -1.23948900 0.01786600C -2.82102500 0.11311100 -0.07102100C -4.16466400 0.56656400 -0.06733000C -5.24211900 -0.41175700 0.02674500H -2.05157500 0.87026600 -0.16950900O -4.51745600 1.79943500 -0.13415900O -6.48818300 0.04342600 0.02520500H -6.43368800 1.01895900 -0.05486600H -5.76330700 -2.49328100 0.19732100H -3.38821200 -3.23244100 0.18251500C -1.19125400 -1.78949900 0.01345700H -1.11782400 -2.87344100 0.03308600C -0.03646200 -1.09893700 0.00470900H 0.00725600 -0.01529000 0.01182100C 1.25667300 -1.82026300 0.00278500O 1.39067800 -3.03278500 -0.00419000O 2.28458900 -0.94952300 -0.00374700C 3.61749200 -1.53847100 -0.02331800H 3.70915400 -2.20076400 0.84482000H 3.71809300 -2.15321800 -0.92375900C 4.61666900 -0.42690700 0.00900600C 5.13514400 0.04057400 1.25572400H 4.81669900 -0.43358100 2.19617800C 6.04719500 1.09376100 1.28471300H 6.43807000 1.43803200 2.25107800C 6.45315300 1.69994200 0.07516800H 7.16618000 2.51767500 0.09080300C 5.95258600 1.25015900 -1.17130800H 6.27218600 1.70770000 -2.12272800C 5.03692200 0.18819400 -1.19759900H 4.64510200 -0.15958600 -2.16306900H -3.45764500 2.65864100 -0.43680900O -2.34161600 3.43951600 -0.77108800O -2.01498700 4.01169500 0.52950400H -2.31678800 4.92763300 0.39757100
xi. The transition state coordinates of reaction involving BBC3 + HO2● in water
C 2.55813200 -1.29423400 0.08702900C 2.79012500 0.05331900 0.34230900C 4.11236600 0.58058300 0.20924300C 5.21706600 -0.35030000 -0.04188000C 4.98805000 -1.72030500 -0.21513700C 3.68612600 -2.18318500 -0.17233600H 1.97316300 0.72124700 0.65476800O 4.44643600 1.82613900 0.30431900H 3.35886200 2.60176900 0.40728400O 6.45076400 0.13730500 -0.09435300H 6.41710100 1.11421300 0.04232700H 5.82342900 -2.38785100 -0.39683600H 3.48974700 -3.23214200 -0.32247700C 1.21485900 -1.86699300 0.06920000H 1.15403700 -2.95258000 0.05978000C 0.05884600 -1.16725800 0.02125600H 0.02764900 -0.07417700 -0.01109400C -1.24371500 -1.86579000 -0.02254500O -1.40175700 -3.07264400 -0.04595700O -2.26392300 -0.98050100 -0.01617700C -3.62004200 -1.54602000 -0.04312400H -3.71398400 -2.13675200 -0.95759700H -3.71918900 -2.19788800 0.82059900O 2.12725000 3.32960000 0.48951600O 2.18488900 4.14779500 -0.86017000

11
H 2.57256200 5.05766000 -0.52089800C -4.59816200 -0.41287200 -0.01076400C -5.07587500 0.08696900 1.20778000C -5.07034100 0.16242900 -1.20893700C -5.98630600 1.14730500 1.24923000H -4.71936400 -0.35628500 2.14371600C -5.99052100 1.21313500 -1.17063400H -4.71949100 -0.22629000 -2.15626100C -6.44978100 1.70748400 0.04930600H -6.34429400 1.51608100 2.19678600H -6.34401600 1.64656200 -2.10768000H -7.16046500 2.52928300 0.08020000
xii. The transition state coordinates of reaction involving BBC4 + HO2● in vacuum
C -4.17190700 1.96571800 0.00429500C -2.83479100 2.29886600 0.00194000C -1.81758400 1.30449300 -0.00043600C -2.20968600 -0.05421800 -0.00050300C -3.54618300 -0.40550200 0.00167600C -4.58263400 0.60248500 0.00419900H -1.45762200 -0.83892200 -0.00260000O -4.00405000 -1.68289300 0.00139200H -3.27210200 -2.31475700 -0.00010700O -5.83423200 0.29513900 0.00623100H -4.94955000 2.72168200 0.00611900H -2.54091600 3.34452100 0.00189800C -0.43293500 1.73154500 -0.00310000H -0.26462400 2.80717400 -0.00506900C 0.67435000 0.95201000 -0.00347300H 0.63855100 -0.13219000 -0.00114700C 2.01629500 1.56440800 -0.00716200O 2.25694700 2.76070900 -0.01094300O 2.97951500 0.60928700 -0.00593400C 4.35220500 1.09296800 -0.01060200H 4.49448100 1.71319200 -0.90006000H 4.49700600 1.72533500 0.86973100C 5.26969900 -0.09904100 -0.00346000C 5.68628000 -0.68406000 -1.20704200H 5.34877700 -0.26409100 -2.15127900C 6.52979600 -1.79702300 -1.20235700H 6.84756800 -2.23922000 -2.14219000C 6.96891800 -2.33634000 0.01027900H 7.62882700 -3.19907700 0.01548900C 6.56125700 -1.75841200 1.21592100H 6.90346800 -2.17041000 2.16088300C 5.71726200 -0.64565700 1.20686700H 5.40392600 -0.19586100 2.14567400H -6.31852900 -1.04084700 0.00364400O -6.97319200 -2.37191000 0.00119100O -8.33586400 -2.03492100 0.00273400H -8.75430900 -2.91324900 -0.00061200
xiii. The transition state coordinates of reaction involving BP3HC + HO2● in vacuum
C 5.68834000 -2.21519600 0.65230800C 4.29868700 -2.41439800 0.59094000C 3.40440500 -1.33528700 0.38112200C 3.93357200 -0.05833100 0.21678200C 5.34687200 0.16826600 0.28024300C 6.21971100 -0.95047900 0.51222800H 3.30295300 0.80806700 0.04475400O 5.85355300 1.34025800 0.12439300H 7.28592200 -0.75910900 0.56287000H 6.34222500 -3.06580600 0.81787400H 3.89504600 -3.41607700 0.70549400C 1.96752600 -1.62378700 0.33674600H 1.68326700 -2.67036900 0.43091700C 0.96404300 -0.74142500 0.19884600H 1.11854600 0.32819500 0.10335400C -0.43703800 -1.22026800 0.17737800O -0.79135000 -2.38070300 0.26741800O -1.29361000 -0.18162300 0.04339700C -2.69623000 -0.52287300 0.01074600H -2.89119700 -1.16415100 -0.85483100H -2.94955800 -1.09622200 0.90779300C -3.48288400 0.78387800 -0.06744400H -3.16215000 1.33520700 -0.95793400

12
H -3.22581500 1.40018000 0.80103000C -4.97530000 0.53121600 -0.11279100C -5.64167900 0.38805000 -1.33682300H -5.08335400 0.49208600 -2.26416900C -7.01075000 0.12262300 -1.38004700H -7.51053300 0.01994100 -2.33901000C -7.73662000 -0.00561400 -0.19501100H -8.80292800 -0.20910000 -0.22675600C -7.08479300 0.13434800 1.03123200H -7.64259300 0.04088500 1.95861500C -5.71580500 0.39999600 1.06957700H -5.21548800 0.51378500 2.02839700H 4.82854200 2.44162300 0.17801500O 3.81074200 3.41926200 0.24761600O 3.76262300 3.81460000 -1.12402600H 3.53265700 4.75333700 -1.02562600
xiv. The transition state coordinates of reaction involving BP4HC + HO2● in vacuum
C 5.23398900 -0.52912700 -0.02285700C 3.93184100 -0.96333700 -0.04975500C 2.83885100 -0.05279000 -0.04223100C 3.12960200 1.34444200 -0.02193900C 4.41978400 1.80279700 -0.00984900C 5.52743200 0.88032200 -0.00446200H 2.31224200 2.05818900 -0.02182500H 4.65122200 2.86243900 -0.00161800O 6.72441100 1.33345300 0.02241300H 6.07059000 -1.22114900 -0.02037200H 3.71904400 -2.02868400 -0.06598600C 1.49786300 -0.58147200 -0.04929000H 1.40325500 -1.66555800 -0.08050200C 0.33441600 0.11392000 -0.01756000H 0.28852500 1.19677500 0.02049500C -0.95369300 -0.60891900 -0.02767500O -1.08457000 -1.81827600 -0.07508000O -1.99089700 0.25897800 0.02634900C -3.30703100 -0.33704200 0.03094300H -3.45094500 -0.89871600 -0.89757800H -3.37701900 -1.04875200 0.85924100C -4.32376000 0.79398700 0.16898400H -4.18875000 1.49367900 -0.66304000H -4.11130800 1.34646400 1.09085500C -5.74169700 0.26181300 0.18737300C -6.46614600 0.10932200 -1.00206000H -6.01535400 0.41114400 -1.94452700C -7.75812800 -0.41710200 -0.99043300H -8.30592200 -0.52372100 -1.92230600C -8.34661100 -0.80083900 0.21545000H -9.35335000 -1.20792000 0.22666000C -7.63590900 -0.65327600 1.40760000H -8.08813100 -0.94477100 2.35118400C -6.34433100 -0.12647800 1.39123000H -5.79779300 -0.00961100 2.32408200H 7.74637600 0.25438700 -0.18057500O 8.70861200 -0.76490400 -0.42603600O 9.21853500 -0.92990900 0.89671900H 10.08003900 -1.33302200 0.69878300