Rutherford model of the atom
-
Upload
saharshjain -
Category
Education
-
view
5.723 -
download
18
description
Transcript of Rutherford model of the atom

Ernest Rutherford (1871-1937)
• Learned physics in J.J. Thomson’ lab.
• Noticed that ‘alpha’ particles were sometime deflected by something in the air.
• Gold-foil experiment
Rutherford
PAPER
Rutherford
PAPER
Animation by Raymond Chang – All rights reserved.

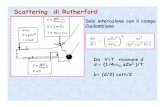
Rutherford ‘Scattering’
• In 1909 Rutherford undertook a series of experiments• He fired (alpha) particles at a very thin sample of gold foil• According to the Thomson model the particles would only
be slightly deflected• Rutherford discovered that they were deflected through large
angles and could even be reflected straight back to the source
particlesource
Lead collimator Gold foil

Rutherford’s Apparatus
beam of alpha particles
radioactive substance
gold foil
circular ZnS - coated
fluorescent screen
Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
Rutherford received the 1908 Nobel Prize in Chemistry for his pioneering work in nuclear chemistry.

Rutherford’s Apparatus
Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
beam of alpha particles
radioactive substance
fluorescent screencircular - ZnS coated
gold foil

Geiger-Muller Counter
Speaker gives“click” for
each particle
Window
Particlepath
Argon atoms
Hans Geiger

Geiger Counter
e-
e-e-
e-+ +
++
Metal tube(negatively charged)
Ionization of fill gastakes place alongtrack of radiation
Ionizingradiationpath
Window
Atoms or moleculesof fill gas
Central wire electrode(positively charged)
Wilbraham, Staley, Matta, Waterman, Chemistry, 2002, page 857
Free e- are attracted to(+) electrode, completing the circuit and generating a current. The Geiger counter then translates the current reading into a measure of radioactivity.
Speaker gives “click” for
each particle(+)
(-)

What he expected…

What he got…richochetingalpha particles

The Predicted Result:
expected path
expected marks on screen
mark onscreen
likely alphaparticle path
Observed Result:

Interpreting the Observed Deflections
Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
.
.
.
.
.
.
.
.
.
.
.
.
.
.
gold foil
deflected particle
undeflected particles
.
.beam ofalpha particles
.

Rutherford Scattering (cont.)
Rutherford interpreted this result by suggesting that the particles interacted with very small and heavy particles
Particle bounces off of atom?
Particle attracts to atom?
Particle goes through atom?
Particle path is alteredas it passes through atom?
.
Case A
Case B
Case C
Case D

Table: hypothetical description of alpha particles
alpha rays don’t diffract
alpha rays deflect towards a negatively charged plate and away from a positively charged plate
alpha rays are deflected only slightly by an electric field; a cathode ray passing through the same field is deflected strongly
... alpha radiation is a stream of particles
... alpha particles have a positive charge
... alpha particles either have much lower charge or much greater mass than electrons
observation hypothesis
(based on properties of alpha radiation)
Copyright © 1997-2005 by Fred Senese

Explanation of Alpha-Scattering Results
Plum-pudding atom
++
+
+
+
+
+
+
-
-
-
-
-
-
-
-
Alpha particles
Nuclear atom
Nucleus
Thomson’s model Rutherford’s model

Results of foil experiment if plum-pudding had been correct.
Electrons scatteredthroughout positive
charges
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 57
++
+
+
+
+
+
+
-
-
-
-
-
-
-
-

Interpreting the Observed Deflections
Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
.
.
.
.
.
.
.
.
.
.
.
.
.
.
gold foil
deflected particle
undeflected particles
.
.beam ofalpha particles
.

Rutherford’sGold-Leaf Experiment
Conclusions:
Atom is mostly empty space
Nucleus has (+) charge
Electrons float around nucleus
Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120

• Hit moth driving car – no change in car direction
• Hit deer – car changes direction
Alpha particle
Large angle of deflection, must have hit massive object!
moth
deerGold Atom