P341 Comparison of Flow Cytometry and High Content ......Spotfire visualisation of cell phenotype...
Transcript of P341 Comparison of Flow Cytometry and High Content ......Spotfire visualisation of cell phenotype...

a
Liz Roquemore*, Elizabeth Price, Katherine Czysz, Catherine Hather, Mike Honeysett and Nick Thomas GE Healthcare, The Maynard Centre, Forest Farm, Whitchurch, Cardiff, Wales, UK CF14 7YT. Tel: +44 (0)29 2052 6499; Fax: +44 (0)29 2052 6230; *e-mail: [email protected]
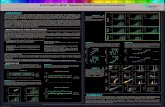
Figure 9: Cell Cycle Analysis. Spotfire DecisionSIte visualisation of nuclear DNA fluorescence (x-axis) and α-Actinin expression (y-axis) data imported directly from IN Cell Investigator Developer Toolbox. Data points are coloured according to Developer decision tree phenotype classification.
Figure 10: Cell Cycle Analysis. DNA content histograms for each α-Actinin/GATA phenotypic class.
Figure 11: Cell Cycle Analysis. DNA content histograms for cardiomyocyte cultures seeded at 40,000 and 20,000 cells/well.
References
1. Thomas N., High-content screening: a decade of evolution. 2010; J Biomol Screen. (1):1-9.
Introduction
IN Cell Analyzer 2000 is a modular benchtop system designed for rapid, high-resolution imaging and analysis of live or fixed biological samples. A 4Mp imaging sensor coupled with objectives from 2x to 100x provides an extremely flexible imaging environment for imaging cytometry analysis of a wide range of cellular systems[1]. High-throughout imaging is supported by automated image analysis in IN Cell Investigator which supports dynamic data linkage to Spotfire DecisionSite™ to enable rapid and efficient visualization and analysis of multi-parameter cellular data. IN Cell Investigator supports Decision Tree data gating allowing data classification during analysis and annotation of Spotfire DecisionSite™ data visualizations. In this study we have explored the use of high content analysis (HCA) for monitoring differentiation of human embryonic stem cells (hESC) to cardiomyocytes and compared the utility of HCA with traditional flow cytometry analysis.
Methods
Cryopreserved cardiomyocytes produced by differentiation of H7 human embryonic stem cells using a proprietary protocol were seeded at densities between 2,500 and 80,000 cells/well in matrigel coated 96 well plates. Cells were fixed and probed using primary antibodies against α-Actinin, Troponin I and GATA-4 in combination with Fluorescein (GATA-4) and Cy5 (α-Actinin & Troponin I) second antibodies. For HCA analysis nuclear DNA was stained with Hoechst and cells were imaged on IN Cell Analyzer 2000 using 2x and 10x objectives. Parallel flow cytometry analysis was carried on trypsinised cells using a FACSCalibur instrument. IN Cell Analyzer images were analyzed using IN Cell
Investigator Developer Toolbox using a custom algorithm to abstract fluorescence marker intensity, DNA fluorescence together with spatial and morphological data. Data was exported directly from Developer Toolbox through a dynamic link allowing fast and efficient visualisation of data and review of phenotype classification.
Results
Identical cultures of cardiomyocytes differentiated from hESC were analysed by flow cytometry and HCA (Figures 1 & 2). IN Cell Analyzer allowed marker expression to be measured in-situ in fixed and stained cultures, providing an improved workflow over flow cytometry by removing the need to trypsinise and process cells for analysis. Analysis of α-Actinin expression showed that flow
cytometry analysis returned a lower percentage of α-Actinin positive cells that HCA analysis. Visual inspection of cultures indicated that flow cytometry may under estimate the percentage of cardiomyocytes, possibly due to cell loss in processing for flow cytometry. To investigate this phenomenon further and to further evaluate the use of IN Cell Analyzer for in-situ evaluation of stem cell differentiation we developed an HCA workflow which allowed generation and visualization of multi-parameter
Figure 1: Analysis of Stem Cell Differentiation to Cardiomyocytes. Cardiomyocytes differentiated from hESC were stained for α-Actinin and analysed by HCA on IN Cell Analyzer (left) and by flow cytometry (right). HCA analysis indicated 20.7% α-Actinin +ve cells; flow cytometry analysis indicated 15.5% α-Actinin +ve cells.
Figure 2: Analysis of α-Actinin Expression in hESC Cardiomyocytes. Spotfire DecisionSite scatterplot visualisation and classification of IN Cell Analyzer α-Actinin expression data from Figure1. α-Actinin +ve cells categorised by Developer Toolbox analysis are shown in green.
cell data with direct linkage to high resolution images. Imaging of cardiomyocyte cultures with a 2x objective
(Figure 3) allowed rapid review of cell distribution in wells and designation of areas for imaging with a 10x objective for phenotypic analysis. Analysis of α-Actinin, Troponin I and GATA 4 expression in Developer indicated the presence of four phenotypic classes (Figure 4) with cells positive and negative for one or both markers. Dynamic linkage of Developer and Spotfire allowed visual
confirmation of phenotypic classification by linking scatterplot data directly to cell images. The Decision Tree gating function within Developer was used to assign each cell analysed to one of four phenotypic classes and display the phenotype distribution automatically in Spotfire (Figure 5). For further confirmation of phenotype assignment image
mapped data visualizations (Figure 6) were produced in Spotfire using nuclear location coordinates from Developer Toolbox analysis. Access to such spatial and morphometric data to allow confirmation of cellular data and phenotype assignment is a key advantage of HCA over flow cytometry in allowing retrospective review and validation of data. Analysis of α-Actinin and GATA-4 expression and phenotype distribution at different cell seeding densities (Figure 7) showed that all four phenotype classes were present at all cell densities, however the relative frequency of the classes changed with seeding density Direct import of Developer decision tree gating
phenotype classification data into Spotfire (Figure 8) showed a decrease in α-Actinin expression between cultures seeded at 40,000 and 20,000 cells/well. To determine whether the four phenotypic classes
identified by α-Actinin and GATA-4 expression had similar cell cycle distributions and whether the change in phenotype distribution with seeding density was related to cell cycle changes DNA fluorescence intensity data was exported to Spotfire (Figure 9).
Figure 6: Image Mapped Data Visualisation. Top; IN Cell Analyzer 2000 image of human stem cell derived cardiomyocytes stained for DNA (blue), GATA-4 (green) and α-Actinin (red). Bottom: Spotfire visualisation of cell phenotype classification based on α-Actinin and GATA4 expression.
Figure 7: Analysis of Effect of Cell Density on Marker Expression. α-Actinin and GATA-4 expression were measured in cells seeded at densities of 2,500-80,000 cells/well of a 96 well plate.
Figure 8: Analysis of Effect of Cell Density on Marker Expression. Cell classifications assigned using decision tree analysis within IN Cell Investigator Developer Toolbox were used to display variance in phenotype distribution at cell seeding densities of 2,500-80,000 cells/well.
CONCLUSIONS
• The IN Cell Analyzer 2000 high-content imaging system allowed rapid imaging and analysis of marker expression in cells cultured in 96 well plates
• Direct connection of IN Cell Investigator Developer Toolbox with Spotfire DecisionSite™ enabled rapid and efficient visualisation and analysis of multi-parameter cellular data for phenotype classification and cell cycle analysis.
• HCA provides a flexible and efficient alternative analytical approach to flow cytometry with added advantages of recording spatial and morphometric data.
Separation of DNA content data by phenotypic class (Figure 10) showed similar cell cycle distributions for each phenotypic class. Examination of cell cycle data for cells cultured at 40,000 and 20,000 cells/well (Figure 11) did not show any significant differences in cell cycle distribution between the two cell densities. Examination of a range of morphological parameters did not reveal any significant differences in cell morphology between cells cultured at different densities (data not shown).
Figure 3: IN Cell Analyzer 2000 Whole Well Imaging. Cardiomyocytes seeded in 96 well plates at varying densities were fixed and stained for α-Actinin and imaged using a 2x objective and the IN Cell Analyzer 2000 large camera (2048 x 2048 pixels).
Figure 4: Image Linked Data Visualisation. Data from IN Cell Investigator analysis of Cardiomyocytes stained for α-Actinin and GATA-4 imaged on IN Cell Analyzer 2000 using a 10x objective were imported directly into Spotfire DecisionSite for visualisation and analysis. Dynamic data linkage allowed matching of data in Spotfire visualisations (left) with cell images within IN Cell Investigator Developer Toolbox (right). A representative α-Actinin+ve/GATA-4+ve cell is shown (arrow).
Figure 5: α-Actinin and GATA 4 Expression. Decision tree analysis was used within IN Cell Investigator Developer Toolbox to classify and display cell populations following direct data import for visualisation in Spotfire: Purple α-Actinin+ve/GATA-4+ve Red α-Actinin+ve/GATA-4-ve Green α-Actinin-ve/GATA-4+ve Blue α-Actinin-ve/GATA-4-ve
Comparison of Flow Cytometry and High Content Analysis for Stem Cell Research
© 2010 General Electric Company - All rights reserved. GE and GE Monogram are trademarks of General Electric Company. All goods and services are sold subject to terms and conditions of sale of the GE Healthcare company which supplies them. A copy of these terms and conditions are available on request. GE Healthcare reserves the right, subject to any regulatory approval if required, to make changes in specifications and features shown herein, or discontinue the product described at any time without notice or obligation. Contact your GE Healthcare representative for the most current information and a copy of the terms and conditions. The IN Cell Analyzer 1000 and associated analysis modules are sold under use licenses from Cellomics Inc. under US patent numbers US 5989835, 6416959, 6573039, 6620591, 6671624, 6716588, 6727071, 6759206, 6875578, 6902883, 6917884, 6970789, 6986993, 7060445, 7085765, 7117098; Canadian patent numbers CA 2282658, 2328194, 2362117, 2381334; Australian patent number AU 730100; European patent numbers EP 0983498, 1095277, 1155304, 1203214, 1348124, 1368689; Japanese patent numbers JP 3466568, 3576491, 3683591 and equivalent patents and patent applications in other countries. GE Healthcare UK Limited Amersham Place Little Chalfont Buckinghamshire HP7 9NA UK. GE Healthcare Bio-Sciences AB SE-751 84 Uppsala Sweden. GE Healthcare Bio-Sciences Corp. 800 Centennial Avenue PO Box 1327 Piscataway NJ 08855 USA. GE Healthcare Europe GmbH Munzinger Str. 5 D-79111 Freiburg, Germany. This poster was presented at CYTO 2010: XXV Congress of the International Society for Advancement of Cytometry, May 8-12, 2010, Seattle, WA, USA. *To whom all correspondence should be addressed.
P341




![Glucose Metabolism and AMPK Signaling Regulate ...bionmr.unl.edu/files/publications/128.pdf(Molecular Probes). Flow cytometry was performed as de-scribed previously [10, 30, 39, 40].](https://static.fdocument.org/doc/165x107/5f481d3d3b482616b93519a1/glucose-metabolism-and-ampk-signaling-regulate-molecular-probes-flow-cytometry.jpg)












