Neonatal paternal deprivation impairs social recognition and alters levels of oxytocin and estrogen...
Click here to load reader
Transcript of Neonatal paternal deprivation impairs social recognition and alters levels of oxytocin and estrogen...

Hormones and Behavior 65 (2014) 57–65
Contents lists available at ScienceDirect
Hormones and Behavior
j ourna l homepage: www.e lsev ie r .com/ locate /yhbeh
Neonatal paternal deprivation impairs social recognition and alters levelsof oxytocin and estrogen receptor α mRNA expression in the MeA andNAcc, and serum oxytocin in mandarin voles
Yan Cao a, Ruiyong Wu a, Fadao Tai a,⁎, Xia Zhang b, Peng Yu a, Xiaolei An a, Xufeng Qiao a, Ping Hao a
a Institute of Brain and Behavioral Sciences, College of Life Sciences, Shaanxi Normal University, Xi'an, Shaanxi 710062, Chinab University of Ottawa, Institute of Mental Health Research, Ottawa, Ontario K1Z 7K4, Canada
⁎ Corresponding author. Fax: +86 29 85308436.E-mail address: [email protected] (F. Tai).
0018-506X/$ – see front matter © 2013 Elsevier Inc. All rihttp://dx.doi.org/10.1016/j.yhbeh.2013.11.005
a b s t r a c t
a r t i c l e i n f oArticle history:Received 9 May 2013Revised 17 November 2013Accepted 19 November 2013Available online 27 November 2013
Keywords:Mandarin vole (Microtus mandarinus)Paternal deprivationSocial recognitionMedial amygdalaNucleus accumbensOxytocin receptorEstrogen receptor α
Paternal care is necessary for the healthy development of social behavior in monogamous rodents and socialrecognition underpins social behavior in these animals. The effects of paternal care on the development of socialrecognition and underlying neuroendocrine mechanisms, especially the involvement of oxytocin and estrogenpathways, remain poorly understood. We investigated the effects of paternal deprivation (PD: father was re-moved from neonatal pups and mother alone raised the offspring) on social recognition in mandarin voles(Microtus mandarinus), a socially monogamous rodent. Paternal deprivation was found to inhibit the develop-ment of social recognition in female and male offspring according to a habituation–dishabituation paradigm.Paternal deprivation resulted in increased inactivity and reduced investigation during new encounters withother animals. Paternal deprivation reduced oxytocin receptor (OTR) and estrogen receptor α (ERα) mRNA ex-pression in themedial amygdala andnucleus accumbens. Paternal deprivation reduced serumoxytocin (OT) con-centration in females, but had no effect on males. Our results provide substantial evidence that paternaldeprivation inhibits the development of social recognition in female and male mandarin voles and alters socialbehavior later in life. This is possibly the result of altered expression of central OTR and ERα and serum OT levelscaused by paternal deprivation.
© 2013 Elsevier Inc. All rights reserved.
Introduction
Early social factors such as the presence of parents and littermatesare very important for healthy behavioral and neuroendocrine develop-ment. Parental care is one of the most important factors in the early so-cial environment because it provides nourishment, warmth, tactilestimulation and protection (Jia et al., 2009). For example, 3 h of mater-nal separation daily for two weeks impairs rats' social recognition atadulthood (Lukas et al., 2011). Presumably because of the female rolein lactation, research typically emphasizes the role of the mother andneglects that of the father during this critical developmental period.However, paternal care plays an important role in brain and behavioraldevelopment (Ovtscharoff et al., 2006), especially in socially monoga-mous mammals such as prairie voles (Microtus ochrogaster) and thecommon marmoset (Callithrix jacchus) (Wang et al., 1998; Ziegler,2000).
Recent studies in monogamous rodents have shown that paternaldeprivation (PD: father was removed from neonatal pups and motheralone raised the offspring) delays the development of offspring, in-creases levels of anxiety, reduces locomotor activity, sociability, parental
ghts reserved.
and alloparental behaviors and inhibits the formation of pair bonding(Ahern and Young, 2009; Jia et al., 2009, 2011; Yu et al., 2012). Inhumans, paternal involvement is positively correlated with cognitivecompetence in school (Kentner et al., 2010; Phares and Compas,1992). However, the long-lasting effects of PD on social recognition inmonogamous rodents and related neuroendocrine mechanisms remainpoorly understood.
All social relationships are dependent on the ability to rememberand recognize conspecifics. Social recognition is therefore critical for re-production, territorial defense and the establishment of dominance hi-erarchies in a natural context (Ferguson et al., 2002). Maternal careinfluences many aspects of the cognitive ability of offspring in adult-hood. For example, in rats, the offspring of mothers that show increasedlicking and grooming exhibit enhanced spatial and non-spatial learningandmemory (Bredy et al., 2003; Liu et al., 2000). Individual variation inmaternal care also contributes to differences in cognitive developmentin humans and primates, as well as rodents (Bornstein, 1985; Liuet al., 2000; Ruddy and Bornstein, 1982; Tamaroff et al., 1986; Zahariaet al., 1996). In contrast, maternal separation impairs social recognitionperformance (Lukas et al., 2011) and exacerbates age-related learningimpairments in adult rats (Meaney et al., 1988, 1991; Oitzl et al.,2000; Tang, 2001). These studies suggest that maternal investment isimportant for cognitive development in offspring. Neonatal paternal

58 Y. Cao et al. / Hormones and Behavior 65 (2014) 57–65
care is also an important early environmental factor that may affectmany aspects of social behavior in monogamous rodents (Ahern andYoung, 2009; Jia et al., 2009, 2011; Yu et al., 2012). Influential paternalbehaviors include huddling, licking and grooming, body nosing, nose–nose contact, nosing-the-nape of the neck, and riding on the father'sback (Reynolds and Wright, 1979) all similar to types of maternalcare. Paternal presence enhances spatial learning and memory andnovel object recognition in adulthood and in a sex-specific way viainfluencing the amount of licking and grooming received by offspringin California mice (Peromyscus californicus) (Bredy et al., 2004). Here,we predict that paternal deprivation affects social recognition inmonogamous mandarin voles.
Social recognition in rodents depends on the detection of olfactorysignals by the main and accessory olfactory systems followinganogenital investigation. Olfactory information is processed by limbicbrain areas including the medial amygdala (MeA) and the lateral sep-tum (Baum and Kelliher, 2009; Richter et al., 2005; Sanchez-Andradeand Kendrick, 2009) and it is not surprising that theMeA and other lim-bic brain regions are involved in social recognition (Choleris et al., 2006;Ferguson et al., 2002; Hammock and Young, 2007; Kogan et al., 2000;Pierman et al., 2008; Ross and Young, 2009; Spiteri et al., 2010). Centraloxytocin (OT) plays important roles in the formation and maintenanceof social recognition (Bamshad et al., 1993; Insel and Shapiro, 1992;Insel et al., 1994). For example, an infusion of a specific OT antagonistinto the brain ofwildtypemice prevents the expression of a socialmem-ory (Young, 2002). Likewise, infusion of oxytocin receptor (OTR) anti-sense oligonucleotides into the MeA of wildtype females impairssocial recognition abilities (Choleris et al., 2007). The socially monoga-mous prairie vole has a higher social recognition ability and higher den-sity of OTR in the nucleus accumbens (NAcc), a region of the brainassociated with reward and reinforcement, compared to meadowvoles, mice and rats (Insel and Shapiro, 1992; Olaza'bal and Young,2006). It is possible that social reward and reinforcement affects socialapproach and subsequently alters social recognition. These data supportthe hypothesis that differences in OTR expression in the MeA and NAccdetermine behavioral traits (Hammock and Young, 2002). Neonatal pa-ternal deprivation significantly alters OT mRNA clusters in theparaventricular hypothalamic nucleus (PVN) in later life (Ahern andYoung, 2009). Here, we propose that PD affects social recognition via al-teration in OTRmRNA expression in theMeA and NAcc, and OT release.
In addition to the possible roles played by the OT system in socialrecognition, estradiol improves social memory in female rats (Hlinák,1993). Estradiol, by binding with estrogen receptor-beta (ERβ) and-alpha (ERα), regulates oxytocin in the PVN and OTRs, respectively(Patisaul et al., 2003; Young et al., 1998). Administration of estrogensto ovariectomized (ovx) mice increases long-term social recognitionmemory (Tang et al., 2005), and bothmain intranuclear estrogen recep-tors, ERα and ERβ, appear to mediate social recognition, with ERαshowing more efficiency than ERβ (Choleris et al., 2003, 2004, 2006;Imwalle et al., 2002; Sánchez-Andrade and Kendrick, 2011; Spiteri andAgmo, 2009). Systemic treatment with ERα or ERβ agonists improvessocial recognition in mice when administered 48–72 h prior to testing(Choleris et al., 2008; Clipperton-Allen et al., 2006), but only the ERα ag-onist rapidly improves performance (within 40 min) (Choleris et al.,2008; Phan et al., 2011). In theMeA,where both ERα and ERβ are highlyexpressed (Mitra et al., 2003), ERα (Young et al., 1998) but not ERβ(Patisaul et al., 2003) is needed for transcription of the OTR gene. ERαregulation of amygdala OTRs (Choleris et al., 2003) is involved in MeAprocessing of olfactory information relevant to social recognition(Beauchamp and Yamazaki, 2003; Dulac and Torello, 2003; Johnston,2003; Kang et al., 2009). We hypothesized that paternal deprivationpossibly alters OTR and ERα mRNA expression in the MeA and NAcc,and subsequently affects social recognition.
The purpose of this study is therefore to determine the longer lastingeffects of PD on social recognition in socially monogamous mandarinvoles and assess whether OTR and ERα mRNA expression in the MeA
and NAcc, and serum OT, are involved in these effects. These experi-ments were designed to shed light on mechanisms underlying the de-velopment of social recognition and its neuroendocrine system insocially monogamous species.
Materials and methods
Subjects
Subjects used in this study were laboratory-reared F1 or F2 genera-tion animals that originated from a wild population in Henan province,China. Every threemonths, mandarin voles are sourced from thewild tomaintain a large genetic pool in the outbred wild mandarin vole colony.Every individual was numbered to avoid inbreeding. Animals weremaintained on a 14:10 h light:dark cycle (lights on at 2000 h) and at atemperature of 25 ± 3 °C with unlimited access to food (carrot andrabbit chow) and provided with water and cotton nesting material inpolycarbonate cages (44 cm × 22 cm × 16 cm). All procedures wereapproved by the Animal Care and Use Committee of Shaanxi NormalUniversity and were in accordance with the Guide for the Care andUse of Laboratory Animals of China.
Treatment groups
Thirty female and 30male adults were randomly arranged into non-sibling breeding pairs. Females usually give birth to two to five pups perlitter and the sex ratio at birth is nearly 1:1 (Tai et al., 1999). The pairswere randomly assigned to two groups: biparental care (PC) consistingof 20 pairs; and paternal deprivation (PD) consisting of 10 pairs. In thePC group all family members were housed together in their homecage and left undisturbed until pups were weaned. For the PD treat-ment, the male parent was removed during the first 24 h followingbirth of the pups and only the mother reared the pups (Jia et al.,2009). All pups were weaned at 23 d of age and the same-sex siblingpair from each litter was housed in one cage. Female andmale offspringfrom each of the two groups were tested as youth-hoods at 45 d of age.
Social recognition test
Female and male mandarin vole offspring from either the PC or PDgroup (32 voles including eight males and eight females from eachgroup) were randomly chosen for the social recognition test at approx-imately postnatal day 45. Stimulus voles during the social recognitiontests for PD or PC groupswere PC-reared voles of the same sex as exper-imental animals. At the beginning of the test, the experimental vole wasplaced in the experiment cage (44 cm × 22 cm × 16 cm) for 10 min inorder to acclimatize. Testing began when a stimulus vole was intro-duced into the experiment cage of each subject (n = 32 per genotype)for a 5-min confrontation. At the end of the 5-min trial, we removed thestimulus animal and returned it to an individual holding cage. We re-peated this sequence for four trials using a 10-min interval between trialsand introduced the same stimulus animal to each experimental vole inall four trials unless otherwise indicated. In a fifth ‘dishabituation’ trial,we introduced a different stimulus animal. Behaviors displayed by theexperimental voles were recorded using a digital video camera and in-cluded body contact (making physical contact with the other individual,including staying together), investigation (sniffing the stimulus), ap-proach (move directly towards the stimulus), attack (actively fighting,including wrestling, biting or scuffling with the other individual), self-grooming (cleaning the fur or scratching), exploration (moving aroundthe cage with the nose near the bedding, sniffing the substrate or stand-ing upon thehind legs and sniffing the air or the cagewall) and inactivity(sitting still). The frequency and total duration of these behaviors werelater scored by a researcher blind to experimental treatment usingNoldus Observe 9.0 (Noldus Information Technology, Wageningen,Netherlands). Dishabituation scores were calculated by subtracting the

Table 1Nucleotide sequences of primers and cycling conditions used for RT-PCR amplification.
Gene Forward primer(5′ to 3′)
Reverse primer(5′ to 3′)
Cyclingconditions
Ampliconsize (bp)
ERα ACGTTGTGCCCCTCTATGAC
CATGAGGCCCTAGATCGTGT
95 °C for 10 s 20360 °C for 30 s72 °C for 10 s
OTR TTCTTCGTGCAGATGTGGAG
CCTTCAGGTACCGAGCAGAG
95 °C for 10 s 18760 °C for 30 s72 °C for 10 s
β-actin AGCCATGTACGTAGCCATCC
CTCTCAGCTGTGGTGGTGAA
95 °C for 10 s 22860 °C for 30 s72 °C for 10 s
59Y. Cao et al. / Hormones and Behavior 65 (2014) 57–65
amount of investigation in trial four from the amount of investigation intrialfive,while habituation scores (investigation in trial oneminus inves-tigation in trial four) were also calculated.
Serum and MeA and NAcc tissue collection
Additional males and females at approximately postnatal day 45were selected from the PC and PD groups to analyze levels of OT andmRNA expression of OTR and ERα. Experimental voles (n = 8) fromthe four groups (Female PC Groups, Female PD Groups, Male PC Groups,Male PD Groups) were deeply anesthetized with sodium pentobarbitaland blood was collected directly from the heart via a terminal bleed.Serum was separated by centrifugation at 4 °C for 30 min and storedat−20 °C for OT assay. To dissect the MeA and NAcc we first sectionedthe brain in a brain mold with a slice width of 1 mm and quickly trans-ferred sections into an RNA safeguard buffer (Omega BioTek, Norcross,USA). TheMeA and NAccwere then dissected on ice, frozen in liquid ni-trogen and stored at −80 °C prior to RNA extraction. Serum and MeAand NAcc tissues were extracted from these voles in the same mannerdescribed above. All samples were collected between 8:00–9:00 h.
Quantification of serum OT
The serum concentration of OT was measured using a vole-specificenzyme-linked immunosorbent assay kit (Shanghai Hushang Biotech-nology, Shanghai, China) following standard procedures. The optimaldilutions of plasma (1:5) were determined using dilution curves (1:1,1:5 and 1:10). First, the prepared sample and the standard were placedin separate plate wells and incubated for 30 min at 37 °C. Second, theplate was washed four times with wash solution, horseradish peroxi-dase (HRP)-conjugate reagentwas added, and the solutionwas incubat-ed for 30 min at 37 °C. Last, after the plate waswashed four more timeswe added Chromogen solutions A and B. After 15 min of incubation at37 °C the reactionwas stoppedusing a stop solution. The optical density(OD) of the sample was determined at 450 nm using a Metertechmicroplate reader (BioTek Instruments,Winooski, USA) after the readerwas zeroed using the blank well. Variation between duplicate measure-ments was less than 5%.
RNA extraction
Total RNA was extracted from the MeA and NAcc tissues usingRNAiso Plus (TaKaRa Biotechnology, Dalian, China) according to themanufacturer's instructions. Isolated RNA was quantified using a Bio-Rad IQ 5 iCycler (Bio-Rad Laboratories, California, USA), and its integritywas confirmed using agarose gel electrophoresis.
Real-time quantitative polymerase chain reaction analysis
Real-time polymerase chain reaction (PCR)was used tomeasure theexpression of OTR and ERα mRNA in the MeA and NAcc of mandarinvoles. Total RNA (0.15 μg in a final volume of 20 μl) was denatured at65 °C for 5 min, cooled for 2 min and reverse transcribed to cDNAusing a PrimeScript RT reagent Kit (TaKaRa Biotechnology, Dalian,China) at 42 °C for 30 min and 70 °C for 15 min. The sample was thencooled in a Gene Amp PCR system 9700 (Applied Biosystems, Shanghai,China). Real-time PCR was completed using the Light Cycler Systemwith SYBR Green I sequence-nonspecific detection (Roche DiagnosticsGmbH; TaKaRa Biotechnology, Dalian, China). Primer sequences andlengths for ERα and OTR mRNA and the reference gene (β-actin) arepresented in Table 1. All primers were designed and synthesized bySangon Biotech (Shanghai, China).
Each PCR reaction was performed in a total volume of 20 μl contain-ing diluted (1/10) cDNA template (2 μl), forward and reverse primers(1 μM each), 10 μl of SYBR Green I master mix (Taq polymerase, reac-tion buffer, MgCl2, SYBR Green I dye and dNTP mix) and water as
needed to reach the total volume. After an initial Taq activation at95 °C for 1 min, Light Cycler PCR was performed using 40 cycles withthe cycling conditions described in Table 1. To verify the purity of theproducts, a melting curve was produced after each run by first holdingthe reaction mixtures at 55 °C for 10 s and then increasing the temper-ature to 95 °C at a rate of 0.1 °C/s. Threshold (Ct) values were deter-mined using the Light Cycler software (Priego et al., 2008). Relativegene expression was quantified based on the efficiency and crossingpoint deviation of an unknown sample versus a control and expressedin comparison to a reference gene (β-actin) (Pfaffl, 2001).
Statistical analyses
Statistical analyses were conducted using SPSS 10.0 (SPSS Inc.,Chicago, USA), and all datawere checked for normality. Social investiga-tion data during habituation trials in the social recognition testwere an-alyzed by two-way ANOVA with repeated measures for trial and withtreatment for each sex. Social investigation in different trials of differentgroups for each sex was analyzed using one-way ANOVA followed byTukey's HSD post-hoc tests. Because there were no significant interac-tions between sex and treatment on habituation, dishabituation scoresand social behaviors during the first trial in the social recognition test,habituation and dishabituation scores were compared using indepen-dent sample t-tests for each sex; social behaviors were comparedusing multivariate tests. Differences in the amount of OTR and ERαmRNA expression and serum OT concentration among groups were an-alyzed using two-way ANOVA followed by post-hoc tests using Tukey'sHSD if significant interactions between treatment and sex were found,otherwise they were compared using independent sample t-tests foreach sex. All data are presented as mean ± SE and significance wasestablished at P b 0.05.
Results
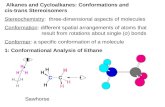
The effect of PD on social recognition in female and male voles
In the habituation–dishabituation paradigm, repeated mea-sures ANOVA did not reveal significant interactions for trial × sex(F4, 140 = 0.88, P = 0.45, η2 = 0.008) and trial × treatment × sex (F4,140 = 0.75, P = 0.52, η2 = 0.007). However, significant interactions be-tween trial and treatmentwere found formales (F4, 140 = 13.17, P b 0.01,η2 = 0.23) and females (F4, 140 = 16.73, P b 0.01, η2 = 0.34). Trials pro-duced significant main effects on investigating in the male (F4, 140 =29.57, P b 0.01, η2 = 0.52) and female (F4, 140 = 18.75, P b 0.01,η2 = 0.38). Post-hoc tests using Tukey's HSD indicates a significant de-crease in investigation between trials in the PC group (P b 0.001 for trial1 vs. trial 3, male: Cohen's d = 3.63, female: Cohen's d = 2.70; and trial1 vs. trial 4, male: Cohen's d = 4.26, female: Cohen's d = 3.71), but in-creased social investigation was found when exposed to a novel animalin trial 5 (trial 4 vs. 5, male: P b 0.001, Cohen's d = 5.62, female:P b 0.001, Cohen's d = 4.09). Animals in the PD group failed to show

60 Y. Cao et al. / Hormones and Behavior 65 (2014) 57–65
any habituation and dishabituation behaviors, spending similaramounts of time investigating the stimulus animal in all five trials anddisplaying impaired social recognition (male: P = 0.49, Cohen'sd = 0.11; P = 0.19, Cohen's d = 0.79; P = 0.159, Cohen's d = 0.77
Fig. 1. Total social investigation of subject voles during a social recognition test including female4; # indicates difference for trial 4 vs. trial 5 revealed by Tukey's HSD test of multiple compariinvestigation on the stimuli at trial 4) in a social recognition test. (d) Dishabituation (total s4) in a social recognition test. * indicates significant differences between two groups revealedby females (e) and males (f) during the first encounter in a social recognition test. * indicate sreared voles; PD: paternal deprivation voles; the significance was established at P b 0.05.
for trial 1 vs. trial 3, trial 1 vs. trial 4 and trial 4 vs. trial 5, respectively;female: P = 0.61, Cohen's d = 0.29; P = 0.69, Cohen's d = 0.19 andP = 0.52, Cohen's d = 0.34 for trial 1 vs. trial 3, trial 1 vs. trial 4 andtrial 4 vs. trial 5, respectively) (Figs. 1a and b).
(a) andmale voles (b). * indicates significant difference for trial 1 vs. trial 3 or trial 1 vs. trialsons. (c) Habituation (total social investigation on the stimuli at trial 1 minus total socialocial investigation on new stimuli minus total social investigation on the stimuli at trialby independent t-test. Total duration of social behavior directed towards a stimulus voleignificant differences between two groups revealed by multivariate test; PC: biparentally-

61Y. Cao et al. / Hormones and Behavior 65 (2014) 57–65
No significant interaction was found between sex and treatmenton dishabituation (F1, 28 = 1.19, P = 0.28, η2 = 0.01) and habituation(F1, 28 = 0.055, P = 0.816, η2 b 0.001) while treatment producedsignificant main effects on dishabituation (F1, 28 = 65.3, P b 0.001,η2 = 0.67) and habituation scores (F1, 28 = 37.42, P b 0.001,η2 = 0.57). Dishabituation and habituation scores in the PC groupwere significantly higher than those in the PD group (dishabituation:male: t14 = 6.04, P b 0.001, Cohen's d = 3.15, female: t14 = 5.38,P b 0.001, Cohen's d = 2.87; habituation: male: t14 = 3.98, P b 0.001,Cohen's d = 1.99, female: t14 = 4.72, P b 0.001, Cohen's d = 2.69)(Figs. 1c and d).
Treatment produced a significant main effect on inactive(F1, 28 = 13.29, P = 0.001, η2 = 0.32) and investigation (F1, 28 =15.62, P b 0.001, η2 = 0.36) behaviors during the first trial. No signifi-cant interaction between sex and treatmentwas found for these behav-iors (P N 0.1, η2 b 0.001). In both males and females, PD significantlyincreased inactivity (male: F1, 14 = 5.55, P = 0.034, Cohen'sd = 1.44; female: F1, 14 = 8.18, P = 0.013, Cohen's d = 1.69), but sup-pressed investigation behavior (male: F1, 14 = 7.25, P = 0.018, Cohen'sd = 1.37; female: F1, 14 = 8.60, P = 0.011, Cohen's d = 1.47). Howev-er, no differences were found for other behaviors (P N 0.05) (Figs. 1eand f).
Serum OT levels in the PC and PD groups
A two-way ANOVA revealed a significant effect of sex (F1, 28 = 11.44,P = 0.002, η2 = 0.23) and treatment (F1, 28 = 5.94, P = 0.021,η2 = 0.12), and a borderline interaction between sex and treatment(F1, 28 = 7.41, P = 0.053, η2 = 0.08) for serum OT levels. PC femaleshad greater serum OT levels than PD females (P = 0.004, Cohen'sd = 1.68); no significant difference was found for males (P = 0.77,
Fig. 2. (a) Serum oxytocin concentration of female and male voles. (b) Oxytocin receptor mRNdifferences between two groups revealed by independent t-test. (c) Estrogen receptorαmRNAin the MeA of female and male voles. In (c) and (d), bars with different letters show significdeprivation voles; the significance was established at P b 0.05.
Cohen's d = 0.62). Serum OT levels in PC females were much higherthan males (P = 0.019, Cohen's d = 1.39), but this difference was notfound in PD voles (Fig. 2a).
OTR and ERα mRNA expression in the MeA and NAcc
The specificity of the primers and the absence of unspecific productswere tested by analyzing the reactions in a 1.5% agarose gel stainedwithethidium bromide. The result showed that only the expected amplifica-tion bandswere shown in the electrophoresis image and no other bandswere visible (Fig. 3). The specificity of the primers was also tested usinga melting-curve analysis. Amplified DNA segments showed consistentpeak Tm values (Fig. 4). The PCR products of the three primer pairswere sequenced and analyzed by Sangon Biotech (Shanghai, China).The resulting sequences showed high similarity with homologoussequences from other rodents including rats, mice and prairie voles.
Both sex and treatment showed significant main effects on the ex-pression of OTR mRNA in the NAcc (sex: F1, 28 = 7.66, P = 0.012,η2 = 0.067; treatment: F1, 28 = 85.87, P b 0.001, η2 = 0.75), ERα(sex: F1, 28 = 32.84, P b 0.001, η2 = 0.31; treatment: F1,28 = 27.72,P b 0.001, η2 = 0.26) and OTR mRNA in the MeA (sex: F1, 28 = 14.10,P = 0.001, η2 = 0.23; treatment: F1, 28 = 13.10, P = 0.001, η2 =0.23). No significant interaction between sex and treatment wasfound for OTR mRNA in the NAcc (F1, 28 = 0.32, P = 0.58,η2 = 0.003). In both males and females, PD reduced levels of OTRmRNA in the NAcc (male: t14 = 6.90, P b 0.001, Cohen's d = 4.76; fe-male: t14 = 6.70, P b 0.001, Cohen's d = 3.72) (Fig. 2b). Significant in-teractions between sex and treatment were found for the expression ofOTR mRNA (F1, 28 = 12.30, P = 0.002, η2 = 0.20) and ERα mRNA intheMeA (F1, 28 = 24.21, P b 0.001, η2 = 0.23). PD reduced the expres-sion of OTR mRNA in the MeA in male offspring (P b 0.001, Cohen's
A expression in the NAcc of female and male voles. In (a) and (b), * indicates significantexpression in theMeA of female and male voles. (d) Oxytocin receptor mRNA expressionant difference revealed by Tukey's HSD test. PC: biparentally-reared voles; PD: paternal

Fig. 3. Agarose gel electrophoresis of the RT-PCR product. 1: OTR band; 2: ERα band; 3: β-actin band; and 4: control (without template).
62 Y. Cao et al. / Hormones and Behavior 65 (2014) 57–65
d = 2.81) and ERα mRNA in the MeA in female offspring (P b 0.001,Cohen's d = 4.10) (Figs. 2c and d). In the PC group, significant sex dif-ferences were found for OTR (P b 0.001, Cohen's d = 2.86) and ERαmRNA expression in the MeA (P b 0.001, Cohen's d = 4.22).
Discussion
Effects of paternal deprivation on social recognition
Using the habituation/dishabituation paradigmwe found that socialinvestigatory behavior in PC offspring followed normal social recogni-tion patterns in which the duration of olfactory investigation declinedacross four exposures to the same individual, and increased when pre-sented with a novel one (Figs. 1a and b) (Ferguson et al., 2002). Howev-er, PD animals failed to show any habituation and dishabituationbehaviors, spending similar amounts of time investigating the stimulusanimal in all five trials, indicating impaired social recognition (Figs. 1cand d). This finding is consistent with previous reports that social
Fig. 4.Melting curve (fluorescence versus temperature) of amplification products. The meltinnonspecific peaks are present.
isolation or maternal separation results in impairments in recognition(Lehmann et al., 1999; Tanaka et al., 2010).
Impairments in social recognition in PD animals in the present studymay be due to the reduction of tactile stimulation such as licking andgrooming from parents. This proposal is supported by a previous reportthat provision of licking-like tactile stimulation to rat pups reduces be-havioral indices of anxiety, improves social learning and novel objectrecognition (Champagne and Curley, 2005; Gonzalez et al., 2001; Weiet al., 2013) and enhances spatial and nonspatial learning and memoryof offspring (Bredy et al., 2003; Liu et al., 2000). The reason may be thattactile stimulation derived from parental licking and grooming appearsto be a critical factor for hippocampal development which plays an im-portant role in recognition (Bredy et al., 2004). Previous work indicatesthat PD animals experience less care, greater exposure and less lickingand grooming in comparison to PC offspring (Ahern and Young,2009). Therefore, PD animals are more likely to display impairment ofsocial recognition.
Interestingly, PD reduced investigative behavior, but increased inac-tive behavior during the first interactionwith a stranger (Figs. 1e and f),agreeingwith one previous report that neonatal PD reduces levels of ex-ploratory, locomotor and social activities (Jia et al., 2009). This may bedue to the lower levels of motivation for exploratory and locomotor ac-tivities induced by PD (Jia et al., 2009, 2011). Reduced motivation forinteracting with a stranger may subsequently inhibit the formation ofsocial recognition in PD animals.
Effects of paternal deprivation on levels of serum OT and OTR mRNAexpression in the MeA and NAcc
PD significantly reduced the expression of OTRmRNA in the NAcc ofboth sex and in the MeA of the male (Figs. 2b and d). This influence ofPD on OTR mRNA expression is in agreement with previous findingsthat biparentally-reared offspring tend to show an increase in OTRbind-ing in the bed nucleus of the stria terminalis (BNST) compared to off-spring reared by single mothers in monogamous prairie vole (Ahernand Young, 2009). Similarly, in forebrain regions, such as the lateral sep-tum, medial preoptic area (MPOA), PVN, BNST, and central amygdala(CeA), OTR binding is increased in adult female rats that receive highlevels of maternal licking and grooming as pups (Champagne et al.,
g temperatures of β-actin, OTR and ERα are 88.5 °C, 92.1 °C and 87.6 °C, respectively. No

63Y. Cao et al. / Hormones and Behavior 65 (2014) 57–65
2001; Francis et al., 2000, 2002). One study in mice also suggests thatmaternal separation (MS) may differently alter levels of OT and its re-ceptor in the PVN, CeA and MeA when females are exposed to femaleand male opponents for the first time in adulthood (Tsuda and Ogawa,2012). Prairie voles which experience early life social deprivationshowed reduced OT receptor binding in brain areas including theNAcc, BNST and lateral septum (Veenema, 2012). Maternal licking andgrooming may play an important role in the regulation of OTR(Francis et al., 2002; Uvnas-Moberg, 1998). Thus, paternal deprivationwhich results in reduced grooming and licking from fathers may subse-quently down-regulate OTR, such as in maternally-deprived animals.
Additionally, PC females had significantly greater serum OT levelsthan PD-reared females, but no difference was found for males(Fig. 2a). During some positive social behavior, OT is coordinatelyreleased into the plasma as well as centrally in females (Churchlandand Winkielman, 2012; Ross et al., 2009a,b). Plasma OT probably re-flects coordinated release by magnocellular cells in the PVN. Thusserum levels of OT may be consistent with levels of OT expression inthe PVN to some extent (Churchland and Winkielman, 2012). Thus,the present data are consistent with the finding that long-term mater-nal separation results in a decrease in OT-ir neurons in the PVN in fe-male mice and rats (Tanaka et al., 2010; Veenema et al., 2007) and PDsignificantly reduces OT-ir in the PVN compared with those reared byboth parents in mandarin voles (Jia et al., unpublished). More OT-positive cells in the parvocellular part of the PVN are found in highlicking and grooming rats compared to low licking and grooming rats(Shahrokh et al., 2010). These previous findings are in agreement withour result that PDoffspring that receive lower levels of parental care dis-play low levels of OT. Difference in the effect of PD on OT levels betweenmales and females may be due to the different roles played by this neu-ropeptide between the sexes.
The reduction in serum OT levels induced by PD co-occurred with adeficit in social recognition in PD animals, and suggests a possible asso-ciation between them. These results are supported by previous findingsthat mice lacking OT or OTR, or mice with deficits in the release of OT(Jin et al., 2007), show significant deficits in social recognition but nor-mal non-social learning and memory (Choleris et al., 2003; Fergusonet al., 2000; Kavaliers et al., 2003; Nishimori et al., 1996). OT also actsin a region-specific manner to modulate social recognition (Bielskyand Young, 2004; Ferguson et al., 2002). For example, OT when admin-istered to the olfactory bulb, the hippocampus, MPOA and amygdala in-fluences social recognition (Bielsky et al., 2005; Ferguson et al., 2002). Infact, injection of OT into the amygdala of OTmutantmice reverses socialrecognition impairments (Ferguson et al., 2001). Thus, it is suggestedthat impaired social recognition induced by PD may be associatedwith a reduction in serum OT levels in females.
OTR mRNA expression in the MeA suppressed by PD in the presentstudymay also contribute to impairment in social recognition. This pro-posal is supported by a previous finding that infusion of OTR antisenseoligonucleotides into the MeA of wild-type females impairs social rec-ognition (Choleris et al., 2007). Similarly, male mice with completelyor partial forebrain OTR knockout also show deficits in social recogni-tion, indicating OTR involvement in social recognition in males(Choleris et al., 2009).
Reduction in OTR mRNA expression in the NAcc may have also con-tributed to the social recognition impairments measured here. Sociallymonogamous prairie voles with better social recognition have higherdensities of OTR in the NAcc than nonmonogamous meadow and mon-tane voles, and pharmacological blockade of those receptors in thisbrain region prevents mating-induced partner preference formation inwhich social recognition is involved (Olaza'bal and Young, 2006;Young et al., 2001). A previous study demonstrated that OTR activationin the NAcc was necessary for the formation of social attachment infemale prairie voles (Young, 2002). The recognition of a familiar individ-ual and the formation of a social memory of that individual is the nextmajor step to forming a social bond. Thus, impairment in social
recognition may be associated with a reduction of OTR mRNA expres-sion in the NAcc.
Effect of PD on levels of ERα mRNA expression in the MeA
ERα mRNA expression in the MeA in female voles was suppressedby PD (Fig. 2c). This finding is consistent with a previous report thatrat pups licked and groomed frequently by theirmothers express higherERα mRNA in the MPOA than females raised by mothers exhibitinglower amounts of maternal care (Boccia and Pedersen, 2001; Franciset al., 2002). Similarly, ERα expression in the BNST, MPOA, ventromedi-al hypothalamic nucleus (VMH) and arcuate nucleus of female offspringis significantly depressed by PD and brief early deprivation (Champagneet al., 2003; Jia et al., 2009). Decreased ERα mRNA expression in theMeA observed in the present study may be partially due to reducedlevels of OT induced by PD. Previouswork has shown that neonatal ma-nipulations of OT in prairie voles result in altered ERα expression in theBNST, MeA, VMH andMPOA (Yamamoto et al., 2006). The ability for OTtreatment to alter the production of ERα mRNA, binding affinity andERα transcriptional activity (Cassoni et al., 2002), suggests that neonataltreatment with OT can alter the expression of ERα by directly alteringthe occurrence of ERα. This is consistent with our result that serumOT levels in PC females were greater than in PD females.
The impairment of social recognitionwe observed inmandarin volesmay be due to a reduction in ERαmRNA expression in theMeA inducedby PD. This is supported by studies using the habituation/dishabituationparadigm that show that ERα-KO male mice are impaired in social rec-ognition and show no behavioral change when a novel mouse is pre-sented (Imwalle et al., 2002). Using a similar paradigm, it has beenshown that both ERα-KO and ERβ-KO female mice are impaired in so-cial recognition and that impairment is similar to that of OT-KO femalemice (Choleris et al., 2003). Furthermore, when tested with amore sen-sitive behavioral paradigm, impairments in social recognition of theERα-KO, OT-KO and ERβ-KO mice were different, with the ERβ-KOmice retaining some degree of social recognition and the ERα-KO andOT-KO mice being completely impaired (Choleris et al., 1998). It is in-ferred that ERα and OT appear to be necessary for social recognition,while ERβ only facilitates it. A specific reduction of ERα in the MeAsuppressed social recognition in female rats using the habituation–dishabituation paradigm (Spiteri et al., 2010), also implying that ERαin the MeA is essential for social recognition.
There is strong evidence that estrogen and OT systems regulate eachother. For example, estrogen, via bindingwith ERα in theMeA, regulatesOTR expression in this brain region (Mitra et al., 2003; Young et al.,1998). It is also well established that through ERβ, estrogens controlOT production in the PVN, and through ERα, they regulate the expres-sion of the OTR gene in the MeA (Choleris et al., 2003), where socially-relevant olfactory information is processed (Beauchamp and Yamazaki,2003; Dulac and Torello, 2003; Johnston, 2003; Kang et al., 2009).According to this model (introduced as a 4-gene ‘micronet’ model byCholeris et al., 2003) the four genes for ERα, ERβ, OT and OTR interplayin the PVN and MeA for normal social recognition to occur. Someresearch also shows that ERα in theMPOAmediates OT and arginine va-sopressin (AVP) involvement in female social recognition (Clipperton-Allen et al., 2012). Thus, interaction between reduced OTR and ERα inthe MeA andNAcc, and reduced serumOT caused by PDmay contributeto the impairment of social recognition in mandarin voles.
Conclusions
Paternal care plays an important role in the development of socialrecognition in offspring. Our results provide substantial evidence thatPD inhibits the formation of social recognition in the socially monoga-mous mandarin vole. These behavioral variations were associatedwith decreases in ERα and OTR mRNA expression in the MeA and

64 Y. Cao et al. / Hormones and Behavior 65 (2014) 57–65
NAcc induced by PD. The reduction of serumOT in PD females may alsobe involved in the inhibition of social recognition.
Acknowledgments
We thank Yu P., Wu R.Y., An X.L. and Qiao X.F. for assistance with ex-periments. This researchwas supported by the National Natural ScienceFoundation of China (31372213 and 31170377), Fundamental ResearchFunds for the Central Universities (GK201305009) and InnovationFunds of Graduate Programs, Shaanxi NormalUniversity (2013CXB010).
References
Ahern, T.H., Young, L.J., 2009. The impact of early life family structure on adult social at-tachment, alloparental behavior, and the neuropeptide systems regulating affiliativebehaviors in the monogamous prairie vole (Microtus ochrogaster). Front. Behav.Neurosci. 3, 17.
Bamshad, M., Novak, M.A., De Vries, G.J., 1993. Sex and species differences in the vaso-pressin innervation of sexually naive and parental prairie voles, Microtus ochrogasterand meadow voles, Microtus pennsylvanicus. J. Neuroendocrinol. 5, 247–255.
Baum, M.J., Kelliher, K.R., 2009. Complementary roles of the main and accessory olfactorysystems in mammalian mate recognition. Annu. Rev. Physiol. 71, 141–160.
Beauchamp, G.K., Yamazaki, K., 2003. Chemical signaling in mice. Biochem. Soc. Trans. 31,147–151.
Bielsky, I.F., Young, L.J., 2004. Oxytocin, vasopressin, and social recognition in mammals.Peptides 25, 1565–1574.
Bielsky, I.F., Hu, S.B., Ren, X., Terwilliger, E.F., Young, L.J., 2005. The V1a vasopressin recep-tor is necessary and sufficient for normal social recognition: a gene replacementstudy. Neuron 47, 503–513.
Boccia, M.L., Pedersen, C.A., 2001. Brief vs. long maternal separations in infancy: contrast-ing relationships with adult maternal behavior and lactation levels aggression andanxiety. Psychoneuroendocrinology 26, 657–672.
Bornstein, M.H., 1985. How infant and mother jointly contribute to developing cognitivecompetence in the child. Proc. Natl. Acad. Sci. U. S. A. 82, 7470–7473.
Bredy, T.W., Humpartzoomian, R.A., Cain, D.P., Meaney, M.J., 2003. Partial reversal of theeffect of maternal care on cognitive function through environmental enrichment.Neuroscience 118, 571–576.
Bredy, T.W., Lee, A.W., Meaney, M.J., Brown, R.E., 2004. Effect of neonatal handling and pa-ternal care on offspring cognitive development in themonogamous Californiamouse.Horm. Behav. 46, 30–38.
Cassoni, P., Catalano, M.G., Sapino, A., Marrocco, T., Fazzari, A., Bussolati, G., Fortunati, N.,2002. Oxytocin modulates estrogen receptor alpha expression and function in MCF7human breast cancer cells. Int. J. Oncol. 21, 375–378.
Champagne, F.A., Curley, J.P., 2005. How social experiences influence the brain. Curr. Opin.Neurobiol. 15, 704–709.
Champagne, F., Diorio, J., Sharma, S., Meaney, M.J., 2001. Naturally occurring variations inmaternal behavior in the rat are associated with differences in estrogen-induciblecentral oxytocin receptors. Proc. Natl. Acad. Sci. U. S. A. 98, 12736–12741.
Champagne, F.A., Weaver, I.C., Diorio, J., Sharma, S., Meaney, M.J., 2003. Natural variationsin maternal care are associated with estrogen receptor α expression and estrogensensitivity in the medial preoptic area. Endocrinology 144, 4720–4724.
Choleris, E., Valsecchi, P., Wang, Y., Ferarri, P., Kavaliers, M., Mainardi, M., 1998. Sociallearning of a food preference in male and female Mongolian gerbils is facilitated bythe anxiolytic chlordiazepoxide. Pharmacol. Biochem. Behav. 60, 575–584.
Choleris, E., Gustafsson, J.A., Korach, K.S., Muglia, L.J., Pfaff, D.W., Ogawa, S., 2003. Anestrogen-dependent four-gene micronet regulating social recognition: a study withoxytocin and estrogen receptor-a and -b knockout mice. Proc. Natl. Acad. Sci. U. S.A. 100, 6192–6619.
Choleris, E., Kavaliers, M., Pfaff, D.W., 2004. Functional genomics of social recognition.J. Neuroendocrinol. 16, 383–389.
Choleris, E., Ogawa, S., Kavaliers, M., Gustafsson, J.A., Korach, K.S., Muglia, L.J., Pfaff, D.W.,2006. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimina-tion: a detailed behavioral analysis with knockout female mice. Genes Brain Behav. 5,528–539.
Choleris, E., Little, S.R., Mong, J.A., Puram, S.V., Langer, R., Pfaff, D.W., 2007. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blockssocial recognition in female mice. Proc. Natl. Acad. Sci. U. S. A. 104, 4670–4675.
Choleris, E., Clipperton, A.E., Phan, A., Kavaliers, M., 2008. Estrogen receptor β agonists inneurobehavioral investigations. Curr. Opin. Investig. Drugs 9, 760–773.
Choleris, E., Clipperton-Allen, A.E., Phan, A., Kavaliers, M., 2009. Neuroendocrinologyof social information processing in rats and mice. Front. Neuroendocrinol. 30,442–459.
Churchland, P.S., Winkielman, P., 2012. Modulating social behavior with oxytocin: howdoes it work? What does it mean? Horm. Behav. 61, 392–399.
Clipperton-Allen, A.E., Cragg, C.L., Wood, A.J., Langmo, A.M., Choleris, E., 2006. Influence ofgonadal hormones on social behavior in male and female mice. Abstr. Soc. Behav.Neuroendocrinol. 10, 22.
Clipperton-Allen, A.E., Lee, A.W., Reyes, A., Devidze, N., Phan, A., Pfaff, D.W., Choleris, E.,2012. Oxytocin, vasopressin and estrogen receptor gene expression in relation to so-cial recognition in female mice. Physiol. Behav. 105, 915–924.
Dulac, C., Torello, A.T., 2003. Molecular detection of pheromone signals inmammals: fromgenes to behaviour. Nat. Rev. Neurosci. 4, 551–562.
Ferguson, J.N., Young, L.J., Hearn, E.F., Matzuk, M.M., Insel, T.R., Winslow, J.T., 2000. Socialamnesia in mice lacking the oxytocin gene. Nat. Genet. 25, 284–288.
Ferguson, J.N., Aldag, J.M., Insel, T.R., Young, L.J., 2001. Oxytocin in the medial amygdala isessential for social recognition in the mouse. J. Neurosci. 21, 8278–8285.
Ferguson, J.N., Young, L.J., Insel, T.R., 2002. The neuroendocrine basis of social recognition.Front. Neuroendocrinol. 23, 200–224.
Francis, D.D., Champagne, F.C., Meaney, M.J., 2000. Variations in maternal behaviour areassociated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol.12, 1145–1148.
Francis, D.D., Young, L.J., Meaney, M.J., Insel, T.R., 2002. Naturally occurring differences inmaternal care are associated with the expression of oxytocin and vasopressin (V1a)receptors: gender differences. J. Neuroendocrinol. 14, 349–353.
Gonzalez, A., Lovic, V., Ward, G., Wainwright, P., Fleming, A., 2001. Intergenerationaleffects of complete maternal deprivation and replacement stimulation on maternalbehavior and emotionality in female rats. Dev. Psychobiol. 38, 11–32.
Hammock, E.A., Young, L.J., 2002. Variation in the vasopressin V1a receptor promoter andexpression: implications for inter- and intraspecific variation in social behaviour. Eur.J. Neurosci. 16, 399–402.
Hammock, E.A.D., Young, L.J., 2007. Neuroendocrinology, neurochemistry, and mo-lecular neurobiology of affiliative behavior. In: Lathja, A., Blaustein, J.D. (Eds.),Handbook of Neurochemistry and Molecular Neurobiology. Springer, NewYork, pp. 247–284.
Hlinák, Z., 1993. Social recognition in ovariectomized and estradiol-treated female rats.Horm. Behav. 27, 159–166.
Imwalle, D.B., Scordakales, E.M., Rissman, E.F., 2002. Estrogen receptor α influencessocially motivated behaviors. Horm. Behav. 42, 484–491.
Insel, T.R., Shapiro, L.E., 1992. Oxytocin receptor distribution reflects social organiza-tion in monogamous and polygamous voles. Proc. Natl. Acad. Sci. U. S. A. 89,5981–5985.
Insel, T.R., Wang, Z.X., Ferris, C.F., 1994. Patterns of brain vasopressin receptor distri-bution associated with social organization in microtine rodents. J. Neurosci. 14,5381–5392.
Jia, R., Tai, F.D., An, S.C., Zhang, X., Broders, H., 2009. Effects of neonatal paternal depriva-tion or early deprivation on anxiety and social behaviors of the adults in mandarinvoles. Behav. Process. 82, 271–278.
Jia, R., Tai, F.D., An, S.C., Zhang, X., 2011. Neonatal paternal deprivation or early depriva-tion reduces adult parental behavior and central estrogen receptor α expression inmandarin voles (Microtus mandarinus). Behav. Brain Res. 224, 279–289.
Jin, D., Liu, H.X., Hirai, H., Torashima, T., Nagai, T., Lopatina, O., Shnayder, N.A., Yamada, K.,Noda, M., Seike, T., Fujita, K., Takasawa, S., Yokoyama, S., Koizumi, K., Shiraishi, Y.,Tanaka, S., Hashii, M., Yoshihara, T., Higashida, K., Islam, M.S., Yamada, N., Hayashi,K., Noguchi, N., Kato, I., Okamoto, H., Matsushima, A., Salmina, A., Munesue, T.,Shimizu, N., Mochida, S., Asano, M., Higashida, H., 2007. CD38 is critical for social be-haviour by regulating oxytocin secretion. Nature 446, 41–45.
Johnston, R.E., 2003. Chemical communication in rodents: from pheromones to individualrecognition. J. Mammal. 84, 1141–1162.
Kang, N., Baum, M.J., Cherry, J.A., 2009. A direct main olfactory projection to the‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromonesfrom males. Eur. J. Neurosci. 29, 624–634.
Kavaliers, M., Colwell, D.D., Choleris, E., Agmo, A., Muglia, L.J., Ogawa, S., Pfaff, D.W., 2003.Impaired discrimination of and aversion to parasitizedmale odors by female oxytocinknockout mice. Genes Brain Behav. 2, 220–230.
Kentner, A.C., Abizaid, A., Bielajew, C., 2010. Modeling dad: animal models of paternalbehavior. Neurosci. Biobehav. Rev. 34, 438–451.
Kogan, J.H., Frankland, P.W., Silva, A.J., 2000. Long-termmemory underlying hippocampusdependent social recognition in mice. Hippocampus 10, 47–56.
Lehmann, J., Pryce, C., Bettschen, D., Feldon, J., 1999. The maternal separation paradigmand adult emotionality and cognition in male and female Wistar rats. Pharmacol.Biochem. Behav. 64, 705–715.
Liu, D., Diorio, J., Day, J.C., Francis, D.D., Meaney, M.J., 2000. Maternal care, hippocampalsynaptogenesis and cognitive development in rats. Nat. Neurosci. 3, 799–806.
Lukas, M., Bredewold, R., Landgraf, R., Neumann, I.D., Veenema, A.H., 2011. Early life stressimpairs social recognition due to a blunted response of vasopressin release within theseptum of adult male rats. Psychoneuroendocrinology 36, 843–853.
Meaney, M.J., Aitken, D.H., van Berkel, C., Bhatnagar, S., Sapolsky, R.M., 1988. Effect of neo-natal handling on age-related impairments associatedwith the hippocampus. Science239, 766–768.
Meaney, M.J., Aitken, D.H., Bhatnagar, S., Sapolsky, R.M., 1991. Postnatal handling attenu-ates certain neuroendocrine, anatomical, and cognitive dysfunctions associated withaging in female rats. Neurobiol. Aging 12, 31–38.
Mitra, S.W., Hoskin, E., Yudkovitz, J., Pear, L., Wilkinson, H.A., Hayashi, S., Pfaff, D.W.,Ogawa, S., Rohrer, S.P., Schaeffer, J.M., McEwen, B.S., Alves, S.E., 2003. Immunolocal-ization of estrogen receptor b in themouse brain: comparisonwith estrogen receptoralpha. Endocrinology 144, 2055–2067.
Nishimori, K., Young, L.J., Guo, Q., Wang, Z., Insel, T.R., Matzuk, M.M., 1996. Oxytocin is re-quired for nursing but is not essential for parturition or reproductive behavior. Proc.Natl. Acad. Sci. U. S. A. 93, 11699–11704.
Oitzl, M.S., Workel, J.O., Fluttert, M., Frosch, F., DeKloet, E.R., 2000. Maternal deprivationaffects behavior from youth to senescence: amplification of individual differencesin spatial learning and memory in senescent brown Norway rats. Eur. J. Neurosci.12, 3771–3780.
Olaza'bal, D.E., Young, L.J., 2006. Oxytocin receptors in the nucleus accumbens facilitate“spontaneous” maternal behavior in adult female prairie voles. Neuroscience 141,559–568.
Ovtscharoff, W.Jr., Helmeke, C., Braun, K., 2006. Lack of paternal care affects synaptic de-velopment in the anterior cingulate cortex. Brain Res. 1116, 58–63.

65Y. Cao et al. / Hormones and Behavior 65 (2014) 57–65
Patisaul, H.B., Scordalakes, E.M., Young, L.J., Rissman, E.F., 2003. Oxytocin, but not oxytocinreceptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus.J. Neuroendocrinol. 15, 787–793.
Pfaffl, M.W., 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45.
Phan, A., Lancaster, K.E., Armstrong, J.N., MacLusky, N.J., Choleris, E., 2011. Rapid effects ofestrogen receptor α and β selective agonists on learning and dendritic spines infemale mice. Endocrinology 152, 1492–1502.
Phares, V., Compas, B.E., 1992. The role of fathers in child and adolescent psychopatholo-gy: make room for daddy. Psychol. Bull. 111, 387–412.
Pierman, S., Douhard, Q., Bakker, J., 2008. Evidence for a role of early oestrogens in thecentral processing of sexually relevant olfactory cues in female mice. Eur.J. Neurosci. 27, 423–431.
Priego, T., Sanchez, J., Pico, C., Palou, A., 2008. Sex-differential expression of metabolism-related genes in response to a high-fat diet. Obesity (Silver Spring) 16, 819–826.
Reynolds, T.J., Wright, J.W., 1979. Early postnatal physical and behavioural developmentof degus (Octodon degus). Lab. Anim. 13, 93–99.
Richter, K., Wolf, G., Engelmann, M., 2005. Social recognition memory requires two stagesof protein synthesis in mice. Learn. Mem. 12, 407–413.
Ross, H.E., Young, L.J., 2009. Oxytocin and the neural mechanisms regulating social cogni-tion and affiliative behavior. Front. Neuroendocrinol. 30, 534–547.
Ross, H.E., Cole, C.D., Smith, Y., Neumann, I.D., Landgraf, R., Murphy, A.Z., Young, L.J.,2009a. Characterization of the oxytocin system regulating affiliative behavior in fe-male prairie voles. Neuroscience 162, 892–903.
Ross, H.E., Freeman, S.M., Spiegel, L.L., Ren, X.H., Terwilliger, E.F., Young, L.J., 2009b. Variationin oxytocin receptor density in the nucleus accumbens has differential effects onaffiliative behaviors in monogamous and polygamous voles. J. Neurosci. 29, 1312–1318.
Ruddy, M.G., Bornstein, M.H., 1982. Cognitive correlates of infant attention and maternalstimulation over the first year of life. Child Dev. 53, 183–188.
Sanchez-Andrade, G., Kendrick, K.M., 2009. The main olfactory system and social learningin mammals. Behav. Brain Res. 200, 323–335.
Sánchez-Andrade, G., Kendrick, K.M., 2011. Roles of α- and β-estrogen receptors inmouse social recognition memory: effects of gender and the estrous cycle. Horm.Behav. 59, 114–122.
Shahrokh, D.K., Zhang, T.Y., Diorio, J., Gratton, A., Meaney, M.J., 2010. Oxytocin–dopamineinteractions mediate variations in maternal behavior in the rat. Endocrinology 151,2276–2286.
Spiteri, T., Agmo, A., 2009. Ovarian hormones modulate social recognition in female rats.Physiol. Behav. 98, 247–250.
Spiteri, T., Musatov, S., Ogawa, S., Ribeiro, A., Pfaff, D.W., Agmo, A., 2010. The role of theestrogen receptorα in the medial amygdala and ventromedial nucleus of the hypothal-amus in social recognition, anxiety and aggression. Behav. Brain Res. 210, 211–220.
Tai, F.D., Wang, T.Z., Zhang, Y.J., 1999. Study on the breeding and inbreeding avoidance inmandarin voles. Acta Theriol. Sin. 19, 278–283.
Tamaroff, M.H., Nir, Y., Straker, N., 1986. Children reared in a reverse isolation environment:effects on cognitive and emotional development. J. Autism Dev. Disord. 16, 415–424.
Tanaka, K., Osako, Y., Yuri, K., 2010. Juvenile social experience regulates centralneuropeptides relevant to emotional and social behaviors. Neuroscience 166,1036–1042.
Tang, A.C., 2001. Neonatal exposure to novel environment enhances hippocampal-dependent memory function during infancy and adulthood. Learn. Mem. 8, 257–264.
Tang, A.C., Nakazawa, M., Romeo, R.D., Reeb, B.C., Sisti, H., McEwen, B.S., 2005. Effects oflongterm estrogen replacement on social investigation and social memory in ovariec-tomized C57BL/6 mice. Horm. Behav. 47, 350–357.
Tsuda, M.C., Ogawa, S., 2012. Long-lasting consequences of neonatal maternal separationon social behaviors in ovariectomized female mice. PLoS One 7, e33208.
Uvnas-Moberg, K., 1998. Oxytocin may mediate the benefits of positive social interactionand emotions. Psychoneuroendocrinology 23, 819–835.
Veenema, A.H., 2012. Toward understanding how early-life social experiences alteroxytocin- and vasopressin-regulated social behaviors. Horm. Behav. 61, 304–312.
Veenema, A.H., Bredewold, R., Neumann, I.D., 2007. Opposite effects of maternal sep-aration on intermale and maternal aggression in C57BL/6 mice: link to hypotha-lamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology32, 437–450.
Wang, Z., Young, L.J., De Vries, G.J., Insel, T.R., 1998. Voles and vasopressin: a review of mo-lecular, cellular, and behavioral studies or pair bonding and paternal behaviors. Prog.Brain Res. 119, 483–499.
Wei, B., Tai, F., Liu, X., Ma, L., Yang, X., Jia, R., Zhang, X., 2013. Neonatal tactile stimulationalleviates the negative effects of neonatal isolation on novel object recognition, socia-bility and neuroendocrine levels inmale adult mandarin voles (Microtus mandarinus).Physiol. Behav. 112–113, 14–22.
Yamamoto, Y., Carter, C.S., Cushing, B.S., 2006. Neonatal manipulation of oxytocin effectsthe expression of estrogen receptor alpha. Neuroscience 137, 157–164.
Young, L.J., 2002. The neurobiology of social recognition, approach, and avoidance. Biol.Psychiatry 51, 18–26.
Young, L.J., Wang, Z., Donaldson, R., Rissman, E.F., 1998. Estrogen receptor α is essentialfor induction of oxytocin receptor by estrogen. Neuroreport 9, 933–936.
Young, L.J., Lim, M.M., Gingrich, B., Insel, T.R., 2001. Cellular mechanisms of social attach-ment. Horm. Behav. 40, 133–138.
Yu, P., An, S.C., Tai, F.D., Zhang, X., He, F.Q., Wang, J.L., An, X.L., Wu, R.Y., 2012. Theeffects of neonatal paternal deprivation on pair bonding, NAcc dopamine recep-tor mRNA expression and serum corticosterone in mandarin voles. Horm. Behav.61, 669–677.
Zaharia, M.D., Kulczycki, J., Shanks, N., Meaney, M.J., Anisman, H., 1996. The effects of earlypostnatal stimulation on Morris water maze acquisition in adult mice: genetic andmaternal factors. Psychopharmacology (Berl) 128, 227–239.
Ziegler, T.E., 2000. Hormones associatedwith non-maternal infant care: a review ofmam-malian and avian studies. Folia Primatol. 71, 6–21.