Nature Medicine: doi:10.1038/nm · Nogo-A/B-deficient Nature Medicine: doi:10.1038/nm.3934. a! b!...
Transcript of Nature Medicine: doi:10.1038/nm · Nogo-A/B-deficient Nature Medicine: doi:10.1038/nm.3934. a! b!...

Supplementary Fig. 1!
a! b! c!
d! Nogo-A/B-deficient!WT!
eNOS!β-actin!
MLEC!Nogo-B!
Nogo-A/B-deficient !WT!nNOS!
β-actin!
Nogo-B!Aorta!
e!
-9 -8 -7 -6 -5 -40
4080
120160200240280
Diam
eter
(μm
)WTNogo-A/B-deficient
PE (LogM)-12-11-10 -9 -8 -7 -6 -5
04080
120160200240280
Diam
eter
(μm
)
U-46619 (LogM)-8 -7 -6 -5
04080
120160200240280
Diam
eter
(μm
)
S1P (LogM)
0.0
0.5
1.0
1.5
nNOS
/β-a
ctin
(a.u
.)
0.0
0.5
1.0
1.5
eNOS
/β-a
ctin
(a.u
.)
WT Nogo-A/B-deficient
Nature Medicine: doi:10.1038/nm.3934

a! b!
d
microsomes !f!Nogo-B!
HSP90!
LC1!
Calnexin!
WT !Ng-A/B-deficient! Ng-A/B-
deficient !WT !
soluble! fraction !
Supplementary Fig. 2!
c!Control!
myriocin 0.3 nM!
myriocin 1 nM!
myriocin 3 nM!
myriocin 10 nM!
myriocin 30 nM!
e!
0.01 0.1 1 10 1001000
0
30
60
90
120
Dose (nmol/L)
Sphi
ngan
ine
inhi
bitio
n (%
)
d!
0
500
1000
1500
2000
2500To
tal c
eram
ide
(pm
ol*m
g-1*m
l -1)
WT Nogo-A/B-deficient
C16-C
er
C24-C
er
C24:1-Ce
r0
100
200
300
400
pmol*mg-1 *ml-1
C14-C
er
C18-C
er
C18:1-Ce
r
C20-C
er
C20:1-Ce
r
C22-C
er
C22:1-Ce
r
C26-C
er
C26:1-Ce
r
dhC16-Cer
0102030405060
pmol*mg-1 *ml-1 ** **
dh-S1PdhS
ph Sph
S1P
0246850
100
150
pmol*mg-1 *ml-1
0 0.1 0.3 1 3 10 30 100 300 !
HUVEC + myriocin (nM)!
PS!SM!
Sphinganine!
Ceramide!
Nature Medicine: doi:10.1038/nm.3934

Nature Medicine: doi:10.1038/nm.3934

Supplementary Fig. 4 !
a!
d! e! f! g! h!
Nogo-B!
HSP90!
VE-cadherin!
αSMA!
b!
Nogo-B!
HSP90!
VE-cadherin!
αSMA!
c!
α-SMA! Nogo-B!
EC-!
Nog
o-A/
B-de
ficie
nt!
IB4! Nogo-B!
Nog
o-A/
Bf/f!
α-SMA!50 μm!Nogo-B!
SMC
-!N
ogo-
A/B-
defic
ient!
0.0
0.4
0.8
1.2
1.6
Nog
om
RN
A ex
pres
sion
Nogo-A/Bf/f
EC-Nogo-A/B-deficient
0.0
0.4
0.8
1.2
1.6
2.0
αSM
A m
RN
A ex
pres
sion VSMC
EC
0.0
0.4
0.8
1.2
1.6
2.0
VE-c
adhe
rin
mR
NA
expr
essi
on VSMCEC
0.0
0.4
0.8
1.2
1.6
Nog
om
RN
A ex
pres
sion
Nogo-A/Bf/f
SMC-Nogo-A/B-deficient
-9 -8 -7 -6 -5 -40
20
40
60
80
100
% In
itial d
iam
eter
Nogo-A/Bf/f
SMC-NogoA/B-deficientEC-NogoA/B-deficient
Phe (LogM)
Nature Medicine: doi:10.1038/nm.3934

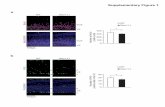
SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. The loss of Nogo-B does not affect the vasoconstriction in response to
pharmacological agents. Concentration-response curves of WT and Nogo-A/B-deficient MA in response to (a)
PE, (n = 8 WT vs. n = 7 Nogo-A/B-deficient), (b) U-46619, (n = 5 per group) and (c) S1P (n = 10 WT vs. n = 6
Nogo-A/B-deficient). WB analysis and relative quantification of (d) eNOS on MLEC from WT and Nogo-A/B-
deficient mice, n = 4 isolations per group, and (e) nNOS on WT and Nogo-A/B-deficient aortas (n = 4 aortas
from 4 mice per group). β-actin was used as loading control. Data were expressed as the mean ± s.e.m.
**P<0.01 compared to WT group. Statistical significance was determined by two-way ANOVA followed by
Bonferroni’s post-test (a–c) and unpaired t-test (d–e).
Supplementary Figure 2. Sphingolipids levels in vascular SMC and SPT assay in EC. WT and SMC-Nogo-
A/B-deficient (a) total ceramide levels and (b) individual sphingolipid species measured by LC/MS as described
in Methods. n = 4 independent VSMC isolation/group; five mice were used for each VSMC isolation. Mean ±
s.e.m. (c) Pharmacological inhibition of SPT activity by increasing concentrations of myriocin in HUVEC. The
inhibition was assessed using [3H]serine as substrate in endothelial cell lysates as described in Methods.
Ceramide-C18 (ceramide), sphinganine, phosphatidylserine (PS) and sphingomyelin-C18:1 (SM). (d) Extracted
lipids were separated by TLC and [3H]sphinganine was detected by using TLC scanner (TLC analyzer RITA,
Raytest Straubenhardt) and reading values were quantified using a standard curve of [3H]serine.
(e) Inhibition of SPT activity, expressed as percent of the control, by increasing concentrations of myriocin. (f) WB analysis for Nogo-B, SPTLC1, Calnexin and HSP90 in microsomes isolated from WT and Nogo-A/B-
deficient mouse lung. Unpaired t-test was performed to evaluate the statistical significance.
Supplementary Figure 3. Effects of W146 and myriocin on vascular tone regulation. (a) Ach cumulative
concentration-response curve in WT mesenteric arteries treated with myriocin (0.3 mg/Kg i.p.) or vehicle in
presence of L-NAME, (left, n = 7 per group), indomethacin, (middle, n = 13 vehicle vs. n = 9 myriocin), and
their combination, (right, n = 5 vehicle vs. n = 6 myriocin). (b) Concentration-response curve of S1P in MA from
WT and Nogo-A/B-deficient mice incubated with L-NAME or vehicle. n = 4 per group. (c) WT mesenteric
arteries treated with W146 (100 nM, 45 min) or vehicle, were incubated with L-NAME and the increase in the
basal tone was measured as internal diameter decrease over the baseline. (n = 11 ctrl vs. n = 5 W146 100
nM). (d) The active diameter of WT mesenteric arteries treated with different concentrations of W146 or vehicle
in response to stepwise increase in intraluminal pressure. (n = 12 ctrl vs. n = 5 W146 100 nM and 1 μM). (e)
Vasoconstriction induced by PE following W146 treatment of WT mesenteric arteries. (n = 8 ctrl vs. n = 9 W146
100 nM). (f) Ach-induced vasodilation in WT mesenteric arteries following W146 (100 nM) treatment. Given
that basal and PE-stimulated tone is different after W146 treatment, the following data are expressed as
Nature Medicine: doi:10.1038/nm.3934

absolute internal diameter (μm) of MA. (n = 21 ctrl vs. n = 7 W146 100 nM). (g) In another set of experiments,
after incubation with W146, Ach cumulative concentration-response curve was performed in presence of: L-
NAME (left, (n = 8 ctrl vs. n = 5 W146 100 nM),.indomethacin (middle, n = 7 per group) and their combination
(right, n = 6 per group). (h) Schematic summary of myriocin and W146 effects on the vascular tone. Data are
expressed as the mean±s.e.m. *P<0.05; **P<0.01 compared to vehicle. Statistical significance was determined
by two-way ANOVA followed by Bonferroni’s post-test (a–b, d–g) and unpaired t-test (c).
Supplementary Figure 4: Characterization of the Cre-mediated excision of Nogo-B in EC and VSMC of EC-
Nogo-A/B-deficient and SMC-Nogo-A/B-deficient mice. (a) IF staining of MA cross-sections for: (top panels)
Nogo-B (red) and α-smooth muscle actin (αSMA; green) in NogoA/Bf/f MA; (central panels) Nogo-B (red) and
EC with isolectin-B4 (IB4, green) in SMC-Nogo-A/B-deficient MA; and (bottom panels) Nogo-B (red) and
αSMA (green) in SMC-Nogo-A/B-deficient MA. (b) Western blot analysis for Nogo-B in EC isolated from
NogoA/Bf/f and EC-Nogo-A/B-deficient lungs. (c) Western blot analysis for Nogo-B in VSMC isolated from
NogoA/Bf/f and SMC-Nogo-A/B-deficient thoracic aortas as described in Methods. Heat shock protein 90
(HSP90) was used as loading control. Vascular endothelial cadherin (VE-cadherin) and αSMA were used as
lineage markers of EC and smooth muscle cells/fibroblasts, respectively. (d) RT-PCR for Nogo-B in EC
isolated from NogoA/Bf/f and EC-Nogo-A/B-deficient lungs; RT-PCR for (e) αSMA and (f) VE-cadherin in
endothelial and smooth muscle cells mRNA isolated from NogoA/Bf/f and SMC-Nogo-A/B-deficient thoracic
aortas as described in Methods. (g) Nogo-B expression of thoracic aorta VSMC mRNA isolated from
NogoA/Bf/f and SMC-Nogo-A/B-deficient mice (h) PE-concentration-response curves of Nogo-A/Bf/f, EC-Nogo-
A/B-deficient and SMC-Nogo-A/B-deficient MA.
Nature Medicine: doi:10.1038/nm.3934

















![5. GENERALIZED METHODS OF MOMENTS (GMM)miniahn/ecn726/cn_gmm.pdf · 5. GENERALIZED METHODS OF MOMENTS (GMM) [1] ... • Estimation of V when the wt are autocorrelated over t: ...](https://static.fdocument.org/doc/165x107/5adebd0d7f8b9aa5088e8359/5-generalized-methods-of-moments-gmm-miniahnecn726cngmmpdf5-generalized.jpg)