!!!Glycolysis, neoglucogenesis, the anaerobic degradation of glucose
Mutualistic degradation of the lignin model compound veratrylglycerol-β-( o ...
Transcript of Mutualistic degradation of the lignin model compound veratrylglycerol-β-( o ...

1654 CAN. I . MICROBIOL. VOL. 21. 1975
7. OZASNE. B.. and J . SAMBROOK. 1971. The biology of oncogenic viruses. Etlitc~tl bv L. G. Silvestri. North- Holland Publishing Company. Amster-dam. pp. 248- 257.
8. SCHEFFLER. I . E., and G. BUTTIN. 1973. Condition- ally lethal mutations in Chinese hamster cells. I. Isola- tion of a temperature-sensitive line and its investiga- tion by cell cycle studies. J . Cell. Physiol. 81: 199-216.
9. si~11.1.~. B. J . . and N. M. WIGGLESWORTH. 1972. A cell-line which is temperat~~re-sensitive for cyto- kinesis. J . Cell. Physiol. 80: 253-260.
10. THOMPSON. L. H . , R. ~ ~ A N K O V I ' ~ % . R. M. BAKER, J .
A. WRIGHT. J . E. T ILL . L. SIMINOVITCH, and G. F. WHITMORE. 1971. Selective and nonselective isola- tion of motlse L-cells and their characterization. J . Cell. Physiol. 78: 431-440.
I I. WOI-LCIAN, Y. . and L. SACHS. 1972. Mapping of sites on the s~11.face membraneof mammalian cells. 11. Rela- tionship of sites fo~.concanavalin A and an ornithine. leucine copolymer. J. Membr. Biol. 10: 1-10.
12. WRIGH I . , J . A. 1973. Evidence forpleiotropic changes in lines of Chinese hamster ovary cells resistant to concanavalin A and phytohe~nagglutinin-P. J. Cell Biol. 56: 666-675.
Mutualistic degradation of the lignin model compound veratrylglycerol-j3-(0-methoxyphenyl) ether by bacteria1
Utrit'er.sity qf Mirrrrt~.sotn, Collegr qf'Bink)gicccI Scic,trcc,s, Fros1111~nte1. Biologictrl Itistitirtr, P.O. Bos 100, Nrrijtrrre, Mirrrresottr 55392
Accepted June 16. 1975
C R ~ \ W F O R D , R. L. 1975. Mutualistic tlegradation of the lignin model compound veratryl- glycerol-P-(o-methoxyphenyl) ether by bacteria. Can. J. Microbiol. 21: 1654-1657.
Complete degradation of the lignin model compo~lnd veratrylglycerol-P-(o-methoxyphenyl) ether is accomplished mutualistically by a two-membered bacterial cult~lre. Bacterial isolate E l , which has been tentatively identified as an A~~itrorc~boctt~t-, grows on veratrylglycerol-P- ((I-methoxyphenyl) ether producing guaiacol (o-methoxyphenol) as a non-metabolizable. bacte- riocidal by-product. When Noccirditr corcrllit~cr (strain A81) is also PI-esent in media containing veratrylglycerol-P-(0-merhoxyphenyl) ether a s the only carbonlenergy source, i t is able to grow on the gl~ai;lcol produced from veratrylglycerol-P-(o-methoxypheny1)ether by isolate E l . Strain A81 alone does not grow on veratrylglycerol-P-(o-methoxyphenyl) ether. In the absence of strain AXI, isolate E l is ~xpidly killed by accumulated guaiacol. In the presence ofthe Noccrrdicr, isolate El maintains its viability.
CRAWFORD. R. L. 1975. Mutualistic degradation of the lignin model compound vemtryl- glycerol-P-(o-methoxyphenyl) ether by bacteria. Can. J . Microbiol. 21: 1654-1657.
La degradation complete d'un compose modele de la lignine, I'ether de veratryl-glycerol- /3-(0-methoxyphenyl). est accomplie mutllellement par une culture bacttrienne contenant deux souches. L'isolat bactkrien E l , lequel a e t i tentativement identifie comme un Acinerobnctrr. be dkveloppe sur le composC modele et produit du gu:~iacol d (o-mithoxyphtnol) comme sous- produit bactericide et non metabolisable. Lorsque tv'ocat.tlici c~ot.tiliitrti (lignee A81) est present sirnultanement dans les milieux contenant le compos6 modele comme seule source de carbone et d'energie, cet organisme est capable de croitre sur le g~laiacol produit par l'isolat E l . La lignee A81. seule, ne peut pas croitre sur le composi modkle. En ['absence de la lignee A81, l'iso- lat E l est rapidement tue par le guaincol accumule. En presence de Nocnrrlirr. l'isolat El maintient sa viabilite.
[Traduit par le journal]
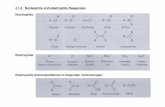
In most instances reported thus far, attack by sults in a t least a transient accumulation of microorganisms on the lignin model compounds guaiacol (compound I11 of Fig. 1) (2, 4-6, 8, 9). veratrylglycerol-P-(o-methoxyphenyl) ether(com- Therefore, during elective enrichment using com- pound I ; Fig. 1) and guaiacylglycerol-j3-(0- pounds I or I1 as enriching substrate (e.g. com- methoxyphenyl) ether (compound 11; Fig. 1) re- pound I, ref. 2) there is likely to be concomitant
enrichment of guaiacol-degrading organisms. In 'Received April 17, 1975. fact, this would probably be of advantage to the
Can
. J. M
icro
biol
. Dow
nloa
ded
from
ww
w.n
rcre
sear
chpr
ess.
com
by
Dep
osito
ry S
ervi
ces
Prog
ram
on
11/1
2/14
For
pers
onal
use
onl
y.

NOTES 1655
H - C - O H OCH H-C-OH OCH
QocH3 0cH33 QocH3 OH
OCH3 OH
I I1 III
FIG. 1. I, Veratrylglycerol-B-(0-rnethoxyphenyl) ether; 11, guaiacylglycerol-a-(0-methoxyphenyl) ether: 111, guaiacol.
whole microbial population since even low levels of guaiacol are often bacteriocidal (unpublished observations).
If attack on compound I by a microorgansim (or microorganisms) produced guaiacol as a non- metabolizable by-product, accumulation of this compound might inhibit continued microbial growth. However, if a second (or more) organism was also present to degrade guaiacol as it is re- leased from compound I, microbial growth might proceed without interruption. The following de- scribes a successful attempt to reproduce such a mutualistic relationship in the laboratory. Veratrylglycerol-P-(0-methoxyphenyl) ether
(compound I) was prepared by the procedure of Adler et 01. (1). Guaiacol (compound 111) was purchased from the Aldrich Chemical Co., Milwaukee, Wisconsin.
Cells were grown in a defined medium con- taining the following in gramsllitre of distilled water: 0.1, KH,PO,; 0.75, K,HPO,; 0.25, NaCI; 1.25, (NH4),S04; 0.01, MgS0,.7H20; and 0.002, FeS0,.7H20. The final pH of the medium was adjusted to 7.0 after addition of substrate (substrate concentrations as ~ndlcated in text). Solid media were prepared by adding 15 gllitre of Noble special agar (Difco) to liquid media.
Stock cultures were maintained on nutrient agar slants, stored at 4 "C, and subcultured every 2 weeks.
Glass, thin-layer chromatography (TLC) plates coated with a 0.1-mm layer of silica gel (Merck HF,,,) were used for analytical chroma- tography. Similar plates were used for prepara- tive TLC, except that a 1.0-mm layer of silica gel was applied to the plates. Developing solvents were (A) benzene - methanol -acetic acid (45 : 8 : 2 by volume), (B) benzene - ethyl acetate (9: 1 by volume), and (C) chloroform-ethanol (19: 1 by
volume). Aromatic compounds on plates were viewed under ultraviolet light. Phenols were de- tected on chromatograms by spraying with a solution of p-nitrobenzene diazonium tetra- fluoroborate (Eastman) in acetone.
Ultraviolet (UV), infrared (IR), and nuclear magnetic resonance (nmr) spectra were de- termined as previously described (3).
Bacterial isolate El was isolated from wood chips by selective enrichment on compound I. El is a non-pigmented, oxidase-negative, immotile, and Gram-negative rod tentatively identified as a species of Acinetobacter. Strain A8 1 was isolated and identified earlier (3) as Nocardia corallitza. Additional characterization of this organism (Ruth Gordon, Institute of Microbiology, Rutgers University; personal communication) indicates that a more satisfactory classification of this bacterium is among bacteria of the rhodochrous complex (7).
Bacterial isolate El grows after initial streak- ing onto agar medium containing 1.0 gllitre of compound I as the only carbon-energy source; however, such cultures invariably become non- viable within 5 to 10 days after inoculation (no growth after transfer into nutrient broth). A strong odor of guaiacol from these plates indi- cates that this compound is an accumulated cata- bolite of veratrylglycerol-P-(0-methoxyphenyl) ether. Suspected guaiacol was extracted from the agar medium with chloroform and isolated by preparative TLC using solvent B. Rf values for the compound suspected to be
guaiacol were identical with those of authentic compound I11 in all four solvent systems. The suspected guaiacol also stained with p-nitro- benzene diazonium tetrafluoroborate in the same manner as authentic guaiacol. The only other major component of the chloroform extract was identical in its chromatographic behavior with authentic compound I. IR and nmr spectra of the isolated compound were determined and were identical with the spectra of authentic compound 111. An equal number of uninoculated control plates carried through the same procedures yielded only unchanged compound I . .
Isolate El grows readily in the defined growth medium when glucose (1.0 gllitre) is supplied as the only carbonlenergy source. Also, El fails to grow in liquid or on solid medium that contains 1 .Og/litre of glucose plus 250 mgllitre of guaiacol. These observations clearly indicate that guaiacol
Can
. J. M
icro
biol
. Dow
nloa
ded
from
ww
w.n
rcre
sear
chpr
ess.
com
by
Dep
osito
ry S
ervi
ces
Prog
ram
on
11/1
2/14
For
pers
onal
use
onl
y.

1656 CAN. J. MICROBIOL. VOL. 21, 1975
is toxic to isolate El at a rather low concentra- tion (0.0259,).
When El was inoculated into liquid medium containing 500 mgllitre ofcompound I as the only carbonlenergy source, it grew to give an optical density (OD,,, ,,,) of about 0.2 units, where growth halted (no further increase in OD,,, ., followed by a slow decrease in OD,,, ",). If the same type of medium was inoculated concom- itantly with E l and strain A81, growth peaked at an optical density of 0.5 units. The Nocardia cannot grow on compound I, but it grows well on guaiacol (3). When such a two-membered culture is streaked onto nutrient agar plates after 7 days of incubation, colonies of both organisms grow. Thus, both organisms maintain viability after prolonged incubation. Therefore, growth of El and strain A81 concomitantly on compound I illustrates a mutualistic relationship. El grows on compound I , producing guaiacol as a non- metabolizable, toxic by-product. When strain A8 1 is also present, this organism is able to grow using the guaiacol released from compound I by attack of E I. The Nocardia thereby detoxifies the growth medium while gaining a source of carbon and energy for growth.
This phenomenon of mutualism was also dem- onstrated on solid medium. Isolate El was streaked onto the center of a plate of agar me- dium containing 1.0 gllitre of compound I as the only carbonlenergy source. The plate smelled strongly of guaiacol after 48 h of incubation at 30 "C. After this preliminary 48-h incubation, N. corallina A8 1 was streaked at several points along the edge of the plate and incubation continued for another 48 h. After this second period of in- cubation, bacterial growth was removed from the agar surface and the plate was flooded with a reagent that stains phenolic compounds (1% FeC1, plus 1% K,Fe(CN), in water). Guaiacol that had diffused outward into the agar from the center of the plate (from the point of El growth) was stained deep blue. The zone of blue covered almost the entire plate surface, except at the points along the plate's edge where there had been inoculations of the Nocardia. Around the points of strain A8 1 inoculation there were clear, unstained areas. Plates inoculated only with E l were stained blue over the entire plate surface, with more pronounced staining near the point of El inoculation. Uninoculated control plates did not stain with the FeCl,/K,Fe(CN), reagent. Guaiacol was the only phenolic compound iden-
tified in chloroform extracts of the agar medium after exposure to E l (see above). ~ h e s e observa- tions show graphically that the Nocardia was de- grading guaiacol as it diffused outward into the vicinity of N. corallina from the central streak of El .
The results summarized above have some relevance to biodegradation of lignin, since com- pound I is a lignin model compound (1). It might be expected that degradation of lignin by soil microorganisms would result in release of toxic, phenolic by-products. These by-products might then be degraded or detoxified by microbes in soil that themselves do not attack the lignin polymer. It is reasonable to expect that degrada- tion of lignin in nature proceeds by the synergis- tic activities of numerous microorganisms, rather than by single species. Exceptions to this general- ization are certain wood-rotting fungi which can degrade lignin without assistance from other species of microorganisms ( 1 0).
Acknowledgments The author thanks T. Kent Kirk of the USDA
Forest Products Laboratory in Madison, Wis- consin, for helpful discussions during the course of this work and the Weyerhaeuser Company Foundation, for financial support.
I. ADLER, E., B. 0. LINDGREN, and U . SAEDEN. 1952. The beta-guaiacyl ether of alpha-veratrylglycerol as a lignin model. Sven. Papperstidn. 55: 245-253.
2. CRAWFORD. R. L., T. K. K I R K , J . M. H A R K I N , and E. McCoy. 1973. Bacterial cleavage of a n arylglycerol-P-aryl ether bond. Appl. Microbiol. 25: 322-324.
3. CRAWFORD. R. L., E. McCoy, J . M. H A R K I N . T. K . K I R K , and J. R . OBST. 1973. Degradation of methoxy- lated benzoic acids by a Nocnrdin from a lignin-rich environment: significance t o lignin degradation and effect of chloro substituents. Appl. Microbiol. 26: 176-184.
4. F U K U Z U M I , T . 1960. Enzymic degradation of lignin. I. Paper-chromatographical separation of intermediate degradation products of lignin by the wood-rotting fungus Poricl srtbncidn Peck Sacc. Bull. Agric. Chem. Soc. Jap. 24: 728-736.
5. F U K U Z U M I , T . , and T. SHIBAMOTO. 1965. Enzymatic degradation of lignin. IV. Splitting of veratryl- glycerol-P-guaiacyl ether by enzymes of Poricr sub- rrcido. J . Jap. Wood Res. Soc. 11: 248-252.
6. F U K U Z U M I , T . , H. TAKATUKA, and K. M I N A M I . 1969. Enzymatic degradation of lignin. V. The effect o f NADH on the enzymic cleavage of the arylglyc- erol-P-guaiacyl ether as a lignin model compound. Arch. Biochem. Biophys. 129: 396-409.
7. GORDON, R. E. 1966. Some strains in search of a genusCorynebncteri~rtt~, Mycobncterilrtt~, Nocardin or what? J. Gen. Microbiol. 43: 329-343.
Can
. J. M
icro
biol
. Dow
nloa
ded
from
ww
w.n
rcre
sear
chpr
ess.
com
by
Dep
osito
ry S
ervi
ces
Prog
ram
on
11/1
2/14
For
pers
onal
use
onl
y.

NOTES 1657
8. ISHIKAWA. H., and T . OKI . 1966. The oxidative deg- Fotnc,s for~~rnrcrrir{s of aromatic compounds structur- radation of lignin. IV. The enzymic hydrolysis ofether ally related to softwood lignin. Arch. Biochem. Bio- linkagesin lignin. J . Jap. WoodRes. Soc.12: 101-107. phys. 100: 140-149.
9. ISHIKAWA, H. , W. J . SCHUBERT, and F. F. NORD. 10. K I R K , T . K. 1971. Effectsof microorganisms on lignin. 1963. investigations on lignins and lignification. XX- Annu. Rev. Phytopathol. 9: 185-210. VIII. The degradation by Polyporrrs ~'crsicolor and
Characterization of a reserve glucan from Megasphaera elsdenii
KOBERT G. BROWN^ A N D BENGT LINDBERG Deportr~~et~t oJOrgcrrlic Chernisrry, Arrhenirrs Lnborcltory, Ur~ir~ersity of S tock l~o l r~~ , S-104 05 S tock I~o l r~~ , Swederl
A N D
K.-J. CHENG Reseorrl~ Sfrrfiott, Agt.icrrlfrrre Ccr71adc1, Lefhhridge. Alberfcr TIJ 4BI
Accepted February 26, 1975
BROWN, R. G., B. LINDBERG, and K.-J. CHENG. 1975. Characterization o f a reserve glucan from Megtrspl~trrrcr elsdet~ii. Can. J. Microbiol. 21: 1657-1659.
A reserveglucan of Megosp/~fleru elsdenii was studied by methylation analysis before and after treatment with isoamylase. The results of thls study indicate that the glucan is of the amylopectin-glycogen type.
BROWN, R. G. , B. LINDBERG et K.-J. CHENG. 1975. Characterization of a reserve glucan from Megtrsphtrerti elsdetlii. Can. J. Micl-obiol. 21: 1657-1659.
On a Ctudii par I'analyse de la mithylation, avant e t aprks traitement avec I'isoamylase, un glucane de reserve obtenu de Megtrsphaertr rlsdenii. Les resultats obtenus demontrent que c e glucan est du type amylopectine-glycogkne.
Reserve glucans containing predominantly a-(1 -, 4) linkages occur frequently in bacteria, particularly in anaerobic organisms. For in- stance, an amylopectin-like a-glucan is produced by Clostridium botulinum (7), and C. pasteuria- num produces an a-(1 -+ 4) linked glucan (1). Recently, Cheng et al. (2) isolated a reserve glucan from Megasphaera elsdenii, one of the rumen bacteria implicated in feedlot bloat (5) , and tentatively characterized it as glycogen. This report will describe further structural eluci- dation of this glucan.
The reserve glucan (glucan I) had [a],,, + 160" after purification as previously described (2) and formed a highly opalescent solution in water which became reddish-brown on addition of iodine. Some of glucan I was further purified by column chromatography using Sephorose 4 B (Fig. 1) to give a homogeneous preparation
'Received October 8, 1974. ZPresent address: Department of Biology, Dalhousie
University, Halifax, Nova Scotia.
[Traduit par le journal]
designated glucan 11. Methylation of both glucan preparations according to the Hakomori pro- cedure (6) followed by hydrolysis yielded the sugars reported in Table 1, which were analyzed by gas-liquid chromatography and mass spectro- metry of their alditol acetate derivatives. These results indicate the occurrence of 4-0- and 4,6-di-0-substituted D-glucopyranose residues. Treatment of glucan I (40 mg) and glucan I1 (17 mg) with a debranching enzyme from Cyto- phaga sp., as described by Gunja-Smith et al. (4), produced reducing groups equivalent to 2.7 and 1.47 mg glucose for glucans I and I1 respec- tively and yielded water-soluble and -insoluble fractions as products (glucan I, 22 and I 1 mg; glucan 11, 10 and 4 mg). The water-insoluble fractions from the M. elsdenii glucan stained reddish-purple with iodine; whereas, the water- soluble fractions gave a reddish-brown color. Methylation analysis (Table I) indicated that the soluble fractions contained linear 4-0-linked residues. Molecular weight determination using columns of Sephadex calibrated with oligo-
Can
. J. M
icro
biol
. Dow
nloa
ded
from
ww
w.n
rcre
sear
chpr
ess.
com
by
Dep
osito
ry S
ervi
ces
Prog
ram
on
11/1
2/14
For
pers
onal
use
onl
y.