Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6...
-
Upload
noah-gardner -
Category
Documents
-
view
220 -
download
0
Transcript of Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6...
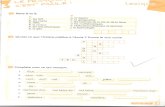
Lecture 18. d-d spectra and MO theory:
3A2g →3T2g
3A2g →1Eg
υ, cm-1
UV
[Ni(NH3)6]2+
visible infrared
The electronic spectra of d-block complexes:
The features of electronic spectra that we need to be able to master are:
1) naming of electronic states and d-d transitions, e.g.3A2g, or 3A2g→1Eg
2) Explanation of relative intensities of bands in the spectra of complexes of d-block metal ions. (The Laporte and spin selection rules)
3) calculation of the crystal field splitting parameters from energies of d-d bands
Naming of electronic states:
In names of electronic states, e.g. 4A2g, the labels A, E, and T, stand for non-degenerate, doubly degenerate, and triply degenerate, while the numeric superscript stands for the multiplicity of the state, which is the number of unpaired electrons plus one. Note that the electronic states can be ground states (states of lowest energy) or excited states:
4A2g
t2g
eg
Multiplicity = 3 unpaired electrons + 1
= 4
Non-degenerateground state =
‘A’
g = gerade
energy
eg eg eg
t2g t2g6A2g
3T2g1A2g
Non-degenerate triply degenerate non-degenerate
Multiplicity= 5 + 1
energy
t2g
Naming of electronic states (contd.):
NOTE: In determining degeneracy, one can re-arrange the electrons, but the number of unpaired electrons must stay the same, and the number
of electrons in each of the eg and t2g levels must stay the same.
Multiplicity= 2 + 1
Multiplicity= 0 + 1
eg eg eg
t2g t2g
5Eg5T2g
2Eg
eg eg eg
t2g t2g
3A2g1Eg
3T2g
Naming of electronic states (contd.):
t2g
t2gground state excited state excited state
ground state excited state ground state
energy
Electronic transitions:
eg eg
eg eg
t2g t2g
t2g t2g
3A2g →3T2g
3A2g →1Eg
3A2g3T2g
3A2g1Eg
ground state excited state
visible infraredUV
green3A2g →3T2g
3A2g →1Eg
[Ni(H2O)6]2+
The electronic spectrum of [Ni(H2O)6]2+:
λ,
The complex looks green, because it absorbs only weakly at 500 nm,the wavelength of green light.
On the previous slide we saw the two bands due to the 3A2g →3T2g and 3A2g →1Eg transitions. The band at λ = 1180 nm which is the 3A2g →3T2g transition shown below, corresponds to Δ for the complex. This is usually expressed as Δ in cm-1 = (1/λ(nm)) x 107 = 8500 cm-1.
The electronic spectrum of [Ni(H2O)6]2+:
eg eg
t2g t2g
3A2g →3T2g3A2g3T2gΔ
= Δ= 8500
cm-1
Note the weak band at 620 nm that corresponds to the 3A2g →1Eg transition. The electron that is excited moves within the eg level, so that the energy does not involve Δ, but depends on the value of P, the spin-pairing energy. The point of interest is why this band is so weak, as discussed on the next slide.
The electronic spectrum of [Ni(H2O)6]2+:
eg eg
t2g t2g
3A2g →1Eg3A2g1EgΔ
= 16100 cm-1
The electronic spectrum of [Ni(H2O)6]2+:
The two peaks at higher energy resemble the 3A2g→3T2g transition, but involve differences in magnetic quantum numbers of the d-orbitals, and are labeled as 3A2g→3T1g(F) and 3A2g→3T1g(P) to reflect this:
3A2g →3T2g
3A2g →3T1g(F)
3A2g →3T1g(P)
3A2g →1Eg
λ,
[Ni(H2O)6]2+
The Selection rules for electronic transitions
There are three levels of intensity of the bands that we observe in the spectra of complexes of metal ions. These are governed by two selection rules, the Laporte selection rule, and the spin selection rule. The Laporte selection rule reflects the fact that for light to interact with a molecule and be absorbed, there should be a change in dipole moment. When a transition is ‘forbidden’, it means that the transition does not lead to a change in dipole moment.
The Laporte Selection rule: This states that transitions where there is no change in parity are forbidden:
g→g u→u g→u u→g forbidden allowed
All transitions within the d-shell, such as 3A2g→3T2g are Laporte forbidden, because they are g→g. Thus, the intensity of the d-d transitions that give d-block metal ions their colors are not very intense. Charge transfer bands frequently involve p→d or d→p transitions, and so are Laporte-allowed and therefore very intense.
The Spin Selection rule: This states that transitions that involve a change in multiplicity (or number of unpaired electrons) are forbidden. This accounts for why transitions within the d-shell such as 3A2g→1Eg that involve a change of multiplicity are much weaker than those such as 3A2g→3T2g that do not.
The Selection rules for electronic transitions
The Selection rules for electronic transitions
3A2g →3T2g
Charge-transfer band – Laporte and spin allowed – very intense
[Ni(H2O)6]2+a
b c
3A2g →1Eg Laporte and spin forbidden – very weak
a, b, and c, Laporteforbidden, spinallowed, inter-mediate intensity
The three types of bands present in e.g. [Ni(H2O)6]2+ are:
1) Laporte-allowed plus spin allowed charge transfer
bands of very high intensity
2) Laporte-forbidden plus spin-allowed d→d transitions (e.g. 3A2g→3T2g) of moderate intensity
3) Laporte forbidden plus spin-forbidden d→d transitions (3A2g→1Eg) of very low intensity.
The Intensity of bands in complexes of d-block ions:
The MO view of electronic transitions in an octahedral complex
t1u*
a1g*
eg*
t2g
t1u
eg
4p
4s
a1g
3d
t2g→t1u*M→L Charge transfer
Laporte and spinallowed
t1u→t2g
L→M Charge transferLaporte and spin
allowed
t2g→eg
d→d transitionLaporte forbiddenSpin-allowed or
forbidden
The eg level in CFTis an eg* in MO
In CFT we consideronly the eg and t2g
levels, which are aportion of the over-
all MO diagram
σ-donor orbitals of six ligands
There are two mechanisms that allow ‘forbidden’ electronic transitions to become somewhat ‘allowed’. These are:
1) Mixing of states: The states in a complex are never pure, and so some of the symmetry properties of neighboring states become mixed into those of the states involved in a ‘forbidden’ transition.
2) Vibronic Coupling: Electronic states are always coupled to vibrational states. The vibrational states may be of opposite parity to the electronic states, and so help overcome the Laporte selection rule.
Why do we see ‘forbidden’ transitions at all?
Mixing of states: Comparison of [Ni(H2O)6]2+ and [Ni(en)3]2+:
[Ni(H2O)6]2+
[Ni(en)3]2+
3A2g →3T2g
3A2g →3T2g(F)
The spin-forbidden 3A2g →1Eg is close to the spin-allowed 3A2g →3T2g(F) and ‘borrows’ intensity by mixing of states
The spin-forbidden 3A2g →1Eg is not closeto any spin allowed band and is very weak
3A2g →1Eg
Note: The two spectra aredrawn on the same graphfor ease of comparison.
Electronic transitions are coupled to vibrations of various symmetries, and the latter may impart opposite parity to an electronic state and so help overcome the Laporte selection rule:
Vibronic coupling:
electronic ground state is ‘g’
electronic excited state is ‘g’
g→g transitionis forbidden
g→(g+u) transitionis allowed
energy
coupled vibrationυ4’ is ‘u’
Electronic transitions, as seenin the spectra of complexes of Ni(II) shown above, are alwaysvery broad because they arecoupled to vibrations. Thetransitions are thus from groundstates plus several vibrationalstates to excited states plusseveral vibrational states (υ1, υ2, υ3),so the ‘electronic’ band is actually a composite of electronic plusvibrational transitions.
υ5
υ3
υ1
υ5’υ3’υ1’
Symmetry of vibrational states, and their coupling to electronic states:
T1u
symmetryvibration
A1g
symmetryvibration
(symbols have same meaning for vibrations: A = non-degenerate, T = triply degenerate, g = gerade, u = ungerade, etc.)
The band one sees in theUV-visible spectrum is thesum of bands due to transitionsto coupled electronic (E) and
vibrational energy levels (υ1, υ2, υ3)
observed spectrum
E E- υ1
E- υ2
E- υ3
E + υ1’E + υ2’
E + υ3’
The spectra of high-spin d5 ions:
6A2g →4T2g
energy
For high-spin d5 ions all possible d-d transitions are spin-forbidden. As a result, the bands in spectra of high-spin complexes of Mn(II) and Fe(III)are very weak, and the compounds are nearly colorless. Below is showna d-d transition for a high-spin d5 ion, showing that it is spin-forbidden.
eg eg
t2g t2g
Complexes of Gd(III) are colorless, while those of other lanthanideM(III) ions are colored, except for La(III) and Lu(III). Why is this?
The spectra of complexes of tetrahedral metal ions:
As we have seen, a tetrahedron has no center of symmetry, and so orbitals in such symmetry cannot be gerade. Hence the d-levels in a tetrahedral complex are e and t2, with no ‘g’
for gerade. This largely overcomes the Laporte selection rules, so that tetrahedral complexes tend to be very intense in color. Thus, we see that dissolving CoCl2 in water produces a pale pink solution of [Co(H2O)6]2+, but in alcohol tetrahedral [CoCl2(CH3CH2OH)2] forms, which is a very intense blue color. This remarkable difference in the spectra of octahedral and tetrahedral complexes is seen on the next slide:
The spectra of octahedral [Co(H2O)6]2+ and tetrahedral [CoCl4]2- ions:
[CoCl4]2-
[Co(H2O)6]2+
The spectra at leftshow the very intensed-d bands in the blue tetrahedral complex[CoCl4]2-, as comparedwith the much weakerband in the pinkoctahedral complex[Co(H2O)6]2+. Thisdifference arises
because the Td com-
plex has no center ofsymmetry, helping toovercome the g→gLaporte selection rule.
![Page 1: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/1.jpg)
![Page 2: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/2.jpg)
![Page 3: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/3.jpg)
![Page 4: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/4.jpg)
![Page 5: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/5.jpg)
![Page 6: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/6.jpg)
![Page 7: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/7.jpg)
![Page 8: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/8.jpg)
![Page 9: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/9.jpg)
![Page 10: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/10.jpg)
![Page 11: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/11.jpg)
![Page 12: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/12.jpg)
![Page 13: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/13.jpg)
![Page 14: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/14.jpg)
![Page 15: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/15.jpg)
![Page 16: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/16.jpg)
![Page 17: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/17.jpg)
![Page 18: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/18.jpg)
![Page 19: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/19.jpg)
![Page 20: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/20.jpg)
![Page 21: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/21.jpg)
![Page 22: Lecture 18. d-d spectra and MO theory: 3 A 2g → 3 T 2g 3 A 2g → 1 E g υ, cm -1 UV [Ni(NH 3 ) 6 ] 2+ visibleinfrared.](https://reader042.fdocument.org/reader042/viewer/2022032612/56649ed25503460f94be1515/html5/thumbnails/22.jpg)