Incomplete cre-mediated excision leads to phenotypic differences between Stra8-iCre; Mov10l1 lox/lox...
Transcript of Incomplete cre-mediated excision leads to phenotypic differences between Stra8-iCre; Mov10l1 lox/lox...

LETTER
Incomplete Cre-mediated Excision leads to PhenotypicDifferences Between Stra8-iCre; Mov10l1lox/lox andStra8-iCre; Mov10l1lox/D mice
Jianqiang Bao,1† Hsiu-Yen Ma,2† Andrew Schuster,1 Yung-Ming Lin,2 and Wei Yan1*
1Department of Physiology and Cell Biology, University of Nevada Reno School of Medicine, Reno, Nevada
2Department of Urology, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Received 20 December 2012; Revised 20 February 2013; Accepted 24 February 2013
Summary: In the Cre–loxp system, expression leveland activity of Cre recombinase in a Cre deleterline are critical because these determine not onlythe cell specificity of gene knockout (KO), but alsothe efficiency of Cre-mediated excision in a specificcell lineage. Although the spatiotemporal expressionpattern of a Cre transgene is usually defined uponthe generation of the mouse line, the Cre excisionefficiency in a specific targeted cell lineage is rarelyevaluated and often assumed to be 100%. Incom-plete excision can lead to highly variable pheno-types owing to mosaicism (i.e., coexistence of cellswith the flox or the recombined flox allele) and thisproblem has long been overlooked. Here, we reportthat Stra8-codon-improved Cre recombinase (iCre),a transgenic allele expressing iCre under the con-trol of the male germ cell-specific Stra8 promoter,could efficiently delete one Mov10l1 flox allele inspermatogenic cells, whereas the excision wasincomplete when two Mov10l1 flox alleles werepresent. The incomplete Cre-mediated excision ledto a testicular phenotype that was much lesssevere than that in the true conditional KO (inacti-vation, 100%) mice. Our findings suggest that it isessential to determine the efficiency of Cre excisionwhen Cre–loxp system is used for deleting genes ina specific cell lineage and the Cre; genelox/D geno-type should be used to evaluate phenotypes insteadof Cre; genelox/lox owing to the fact that the latterusually bears incomplete deletion of the flox al-lele(s). genesis 00:1–10. VC 2013 Wiley Periodicals,Inc.
Key words: conditional gene knockout; Cre; loxp; piRNA;testis; germ line; phenotype; mosaicism
INTRODUCTION
Gene knockout (KO) technologies have greatly facili-tated biomedical research by allowing researchers todetermine the physiological roles of genes during devel-opment and in adult physiology (Gama Sosa et al.,2010; Guan et al., 2010; Yan, 2009). However, manygenes are indispensible for embryonic developmentand global inactivation of these genes leads to embry-onic lethality, and thus precluding further investigationof their functions in adulthood (Aoki and Taketo, 2008;Miller, 2011). To overcome this problem, the Cre–loxpsystem has been widely utilized for inactivating genesin a spatiotemporally regulated manner (Aoki andTaketo, 2008; Speck and Iruela-Arispe, 2009). Generally,this system requires two key components: one is theCre recombinase, the expression of which is driven bya cell lineage- or tissue-specific promoter; the other is agene flanked by two loxp sequences generated by genetargeting (Pluck, 1996). Numerous male germline-spe-cific Cre transgenic mouse lines have been generatedand utilized for producing conditional KO mouselines (Tamowski et al., 2010), including TNAP-Cre,
†Jianqiang Bao and Hsiu-Yen Ma contributed equally to this study.
* Correspondence to: Wei Yan, Department of Physiology and Cell Biol-
ogy, University of Nevada Reno School of Medicine, CMM Room 207B,1664 North Virginia Street, MS0575, Reno, NV 89557, USA. E-mail:
Contract grant sponsor: NIH; Contract grant numbers: HD060858 and
HD071736; Contract grant sponsor: NIH; Contract grant number:P20-RR18751.
Published online in Wiley Online Library (wileyonlinelibrary.com).
DOI: 10.1002/dvg.22389
VC 2013 Wiley Periodicals, Inc. genesis 00:1–10 (2013)

Blimp1-Cre, Vasa-Cre, TSPY-Cre, Kit-Cre, Sycp1-Cre,Pgk2-Cre, Hsp2-Cre, Eno2-Cre, Ngn3-Cre, and Prm1-
Cre (Chung et al., 2004; Gallardo et al., 2007; Ham-mond and Matin, 2009; Inselman et al., 2010; Kido andLau, 2005; Vidal et al., 1998). These mice weredesigned to have Cre expression specifically in malegerm cell lineage in developing (e.g., embryonic, fetaland/or prepubertal) and adult testes, and the actual spa-tiotemporal expression pattern of Cre is mostly eval-uated either by crossing these Cre lines withconditional LacZ or GFP reporter mouse lines (Gallardoet al., 2007; Novak et al., 2000), or based on the detec-tion of Cre mRNA/protein using in situ hybridization orimmunohistochemistry. However, the actual Cre exci-sion efficiency in the targeted cell lineage is rarely eval-uated at genomic levels, and incomplete Cre-mediatedexcision may be responsible for many of the studiesreporting either that some of those Cre lines failed tocompletely delete floxed genes in the testicular germcells (Kimura et al., 2003; Lei et al., 2010; Rasoulpourand Boekelheide, 2006), or a lack of phenotype in thetargeted cell types in which Cre expression is detected.Moreover, discrepancies in phenotypes have often beenobserved among exactly the same Cre–loxp cKO lines(Hayashi et al., 2008; Maatouk et al., 2008), leading toconfusion in functional interpretation of these genes.We have unexpectedly observed that several of the Cremouse lines that we have used displayed abundant Creexpression specifically in the male germ cell lineage,but the efficiency of Cre-mediated excision rarelyreached 100%, rendering a variable proportion of Cre-expressing cells still retaining the functional flox al-lele(s). Here, we use the Stra8-codon-improved Crerecombinase (iCre); Mov10l1
lox/lox line as an exampleto demonstrate this hidden problem. We observed adrastic phenotypic difference between Stra8-iCre;
Mov10l1lox/lox and Stra8-iCre; Mov10l1
lox/D mice, andthe partial Cre-mediated excision appeared to be thecause.
Stra8 (stimulated by retinoic acid 8) is a germline-specific gene exclusively expressed in spermatogonialstem cells (SSCs) in fetal testes, undifferentiated (i.e.,SSCs and prospermatogonia) and differentiated (type A,intermediate, and type B) spermatogonia in postnataltestes (Anderson et al., 2008; Hogarth et al., 2011).Stra8-iCre transgenic mice, generated by inserting a1.4-kb Stra8 promoter region upstream of the iCre-cod-ing sequence, were intended to mimic the endogenousexpression of Stra8 in the spermatogenic cell lineage(Sadate-Ngatchou et al., 2008). By crossing withTg(ACTB-Bgeo/GFP)21Lbe (Z/EG) reporter females(Novak et al., 2000), a previous study has shown thatCre activity can be first detected in prospermatogoniain postnatal day 3 (P3) testes, and Cre expression con-tinues until preleptotene spermatocyte stage (Sadate-Ngatchou et al., 2008).
To verify the spatiotemporal expression of Stra8-
iCre, we first crossed Stra8-iCre males withRosa26mTmG
tg/tg reporter (Muzumdar et al., 2007)females, a double fluorescent Cre reporter transgenicline in which cells without Cre activity express mem-brane-tagged tomato red fluorescence protein (mT)and cells with Cre activity express membrane-taggedeGFP (mG) owing to Cre excision of the floxed STOPcassette. Cryosections of developing Stra8-iCre;
Rosa26mTmG1/tg testes were prepared for imaging
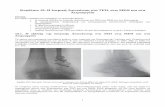
analyses (Fig. 1). In these testes, mG-positive (green)cells represent those with successful Cre excision ofthe floxed STOP cassette, whereas Cre-negative cellsremain red owing to constitutive expression of mT.Previously, we have detected iCre activity first in post-natal day 3 (P3) testes (Wu et al., 2012). Similar to P3testes, only a small proportion of spermatogonia weregreen at P4 and P8, suggesting either iCre was notexpressed or the Cre excision did not occur in thosenongreen cells. At P14, all meiotic cells displayedstrong mG signals within the seminiferous tubules, sug-gesting iCre-mediated excision had peaked in sperma-tocytes. After P21, the highest levels of mG expressionwere detected in round spermatids and pachytenespermatocytes, whereas mG expression in spermatogo-nia and prepachytene spermatocytes was barely detect-able (Fig. 1). The expression patterns of mG suggestthat Stra8-iCre expression starts in a proportion ofspermatogonia around P3 or P4 and continues toincrease with male germ cell development from sper-matogonia to spermatocytes and then spermatids.However, the efficiency of Cre-mediated excision wasmuch lower than 100% in spermatogonia, leading to asignificant proportion of spermatogonia without mGexpression either during testicular development or inadult testes. The iCre-mediated excision reaches full ef-ficiency only in pachytene spermatocytes and sperma-tids in adult testes, whereas the levels of iCre decreaseto the minimum in spermatogonia in the adult testes(Fig. 1). Therefore, the Stra8-iCre line is not appropri-ate for inactivating floxed genes in spermatogonia anda lack of effects/phenotype in spermatogonia whenthis Cre line is used may not necessarily suggest thatthis particular gene does not have an essential role inspermatogonial stage because of the partial Cre-medi-ated excision in spermatogonia.
By crossing Stra8-iCre males with Mov10l1lox/lox
females, we generated Stra8-iCre; Mov10l11/lox mice.
Because of Stra8 expression in spermatogenic cell dur-ing spermatogenesis, sperm from Stra8-iCre;
Mov10l11/lox male are genotypically Stra8-iCre;
Mov10l11/D. We then crossed Stra8-iCre; Mov10l1
1/lox
males with Mov10lox/lox females. The resultant Stra8-
iCre; Mov10l11/D males contained one flox allele and
one recombined flox allele (i.e., KO or delete allele, D)in all of their spermatogenic cells (Fig. 2a). Meanwhile,
2 BAO ET AL.

FIG. 1. Visualization of Stra8-iCre expression and iCre activity in developing testes by crossing the Stra8-iCre line with theRosa26mTmGtg/tg reporter transgenic mouse line. Confocal microscopic analyses of testicular cryosections from Stra8-iCre; mTmG1/tg
mice at postnatal day 4 (P4), P8, P14, P21, and P60, respectively. Representative fluorescent images counterstained with DAPI at variousages are shown and specific membrane-tagged eGFP (mG) signals are observed only within the seminiferous tubules. At P4 and P8, somegerm cells exhibit strong mG signals (arrows), whereas the rest exhibit weak membrane-tagged tomato red (mT) signals (arrowheads). AtP14, germ cells with stronger mG expression are spermatocytes. Levels of mG signals on spermatogonia are much lower compared tothose on spermatocytes and spermatids in P21 and P60 testes. Scale bar 5 50 mm.
MOSAICISM BY PARTIAL CRE-MEDIATED EXCISION 3

by crossing Stra8-iCre; Mov10l11/lox females with
Mov10l1lox/lox males, we obtained Stra8-iCre; Mov10l1-
lox/lox males that have two Mov10l1 flox alleles in all oftheir spermatogenic cells (Fig. 2b). Mov10l1 encodes aputative RNA helicase essential for the biogenesis ofPIWI-interacting RNAs (piRNAs) in the murine germline(Frost et al., 2010; Zheng et al., 2010). Global inactiva-tion of Mov10l1 leads to a phenotype exclusively con-fined to the testis characterized by spermatogenicarrest in leptotene to zygotene transition and male
infertility. Wild-type, flox, and recombined (D) alleleswere genotyped by PCR using two sets of primers (Fig.2c). The first set of primers (FW1 1 Rev) detected thewild-type and floxed allele bands, whereas a second set(FW2 1 Rev) was used to validate the recombinedMov10l1 flox allele (D). Interestingly, testes collectedfrom P60 Stra8-iCre; Mov10l1
lox/D males were muchsmaller in size compared to those of Stra8-iCre;
Mov10l11/lox littermates (controls) (Fig. 2e). Unexpect-
edly, testes derived from P60 Stra8-iCre; Mov10l1lox/lox
FIG. 2. Generation and phenotype of Stra8-iCre; Mov10l11/lox (control), Stra8-iCre; Mov10l1lox/lox (mosaic) and Stra8-iCre; Mov10l1lox/D
(true cKO) mice. (a) Male Stra8-iCre; Mov10l11/lox mice were crossed with Mov10l1lox/lox females to produce Stra8-iCre;Mov10l1lox/D
male mice. (b) Stra8-iCre; Mov10l1lox/lox males were generated by mating female Stra8-iCre; Mov10l11/lox with Mov10l1lox/lox males.(c) Representative PCR gel images showing the different genotypes as indicated. Upper panel: wild-type and flox allele bands weredetected using the first set of primers (FW1 and Rev) at �160 and 400 bp, respectively (arrows), and both bands were absent after Stra8-iCre-mediated excision of Mov10l1 flox allele (D); Lower panel: a �500 bp band indicates the recombined floxed allele (arrowheads).(d) Comparison of the testis weight at postnatal day 60 among the Stra8-iCre; Mov10l11/lox, Stra8-iCre; Mov10l1D/lox and Stra8-iCre;Mov10l1lox/lox males (*P<0.05; **P<0.01). (e) Gross morphology and testis size at postnatal day 60 in Stra8-iCre; Mov10l11/lox and Stra8-iCre; Mov10l1lox/D male mice. (f) Gross morphology and testis size at postnatal day 60 in Stra8-iCre; Mov10l11/lox and Stra8-iCre;Mov10l1-lox/lox male mice (scale bar 5 2 mm).
4 BAO ET AL.

males showed a slight decrease in both weight and sizecompared to those of Stra8-iCre; Mov10l1
1/lox litter-mates (Fig. 2f). A significant difference in testis weightwas also observed between Stra8-iCre; Mov10l1
lox/D
and Stra8-iCre; Mov10l1lox/lox males (Fig. 2d).
To further define the phenotypic differences at histo-logical levels, we analyzed hematoxylin–eosin (HE)-stained, paraffin-embedded cross-sections of Stra8-iCre;
Mov10l1lox/D and Stra8-iCre; Mov10l1
lox/lox testes (Fig.3). Control (Stra8-iCre; Mov10l1
1/lox) male testes dis-play normal spermatogenesis (Fig. 3a), whereas inStra8-iCre; Mov10l1
lox/D males, the most advancedstages of spermatogenic cells within the seminiferousepithelium were those zygotene-like spermatocytes(Fig. 3d, arrows), and all tubules exhibited early meioticarrest at leptotene to zygotene transition withoutexception (Fig. 3c,d), which is consistent with the pre-vious reports on global Mov10l1 KO mice(Mov10l1
2/2) (Frost et al., 2010; Zheng et al., 2010).Interestingly, Stra8-iCre; Mov10l1
lox/lox testes displayedno clear-cut spermatogenic arrest in the seminiferousepithelium (Fig. 3b), as seen in Stra8-iCre; Mov10l1
lox/D
testes (Fig. 3c). As a reflection of spermatogenic disrup-tions, diameters of tubules in Stra8-iCre; Mov10l1
lox/D
testes were much smaller than those of control (Stra8-
iCre; Mov10l11/lox) testes (Fig. 3e). Histologically, nor-
mal seminiferous tubules at various stages of the epithe-lial cycles were often observed in Stra8-iCre;
Mov10l1lox/lox testes but completely absent in Stra8-
iCre; Mov10l1lox/D males (Fig. 3f). Upon examining the
cauda epididymis, we discovered a complete absenceof spermatozoa in Stra8-iCre; Mov10l1
lox/D cauda epidi-dymis, whereas numerous spermatozoa were present inStra8-iCre; Mov10l1
lox/lox cauda epididymis (Fig. 3h)although the total number was drastically reduced com-pared to controls (Stra8-iCre; Mov10l1
1/lox) (Fig. 3g).To further evaluate spermatogenic disruptions, peri-
odic acid-Schiff (PAS)-stained testicular sections wereexamined (Fig. 4). Control (Stra8-iCre; Mov10l1
1/lox)testes (Fig. 4a,b) contained tubules of all 12 spermato-genic stages, whereas cross-sections of Stra8-iCre;
Mov10l1lox/lox testes exhibited a much more heteroge-
neous histology (Fig. 4c–h). Aberrant spermatogenesisappeared to be arrested at late pachytene spermatocyteto round spermatid stages (Fig. 4d,e). Numerous Sertolicell vacuoles were observed, suggesting active sperma-togenic cell depletion (Kyronlahti et al., 2011).Although morphologically normal-looking Stages VI andI seminiferous tubules were present (Fig. 4f,h), a Stage-Itubule displayed a distinct spermiogenic arrest at theelongation step (Steps 9–10) (Fig. 4f,h). Thus, testes ofthe Stra8-iCre; Mov10l1
lox/lox males displayed a muchmore heterogeneous spermatogenic disruptions (Fig.4c–h) compared to those of the Stra8-iCre; Mov10l1
D/
lox males, in which spermatogenesis is uniformlyarrested at leptotene to zygotene transition (Fig. 3c,d).
These results suggest that iCre is more competent tocleave one rather than two flox alleles because the onlydifference between Stra8-iCre; Mov10l1
lox/D and Stra8-
iCre; Mov10l1lox/lox males was one extra flox allele.
Interestingly, we observed similar phenomenon in sev-eral other Cre–flox lines in which either Stra8-iCre orother Cre lines (e.g., Ddx4-Cre or Blimp1-Cre) wasused as the deleter line (data not shown), suggestingthat for most of the Cre lines partial Cre-mediated exci-sion is a common problem when Cre has to deal withtwo flox alleles (lox/lox).
If the Cre excision efficiency was 100%, mice withthe two genotypes, Stra8-iCre; mov10l1
lox/D and Stra8-
iCre; mov10l1lox/lox, should have displayed identical or
at least similar phenotype. The phenotypic differencesmust have resulted from persistence of the flox allele inStra8-iCre; Mov10l1
lox/lox spermatogenic cells owing topartial Cre-mediated excision. To verify this, spermwere collected from the cauda epididymis of Stra8-iCre;
Mov10l1lox/lox males. Abundant sperm were observed
in the epididymis of control (Stra8-iCre; Mov10l11/lox)
male mice (Fig. 5a), whereas no mature sperm werepresent in the cauda epididymis of Stra8-iCre;
Mov10l1lox/D mice (Fig. 5c). In the cauda epididymis of
Stra8-iCre; Mov10l1lox/lox mice, both morphologically
normal and abnormal sperm, as well as depleted roundspermatids are present (Fig. 5b). Those sperm musthave developed from those earlier spermatogenic cells(e.g., spermatogonia, spermatocytes, or spermatids) inwhich one or two flox alleles were not completelydeleted through Cre-mediated excision. To confirm this,sperm were collected from Stra8-iCre; Mov10l1
lox/lox
cauda epididymis followed by DNA extraction and PCR-based genotyping. Indeed, both KO (i.e., recombinedflox allele) and flox alleles were steadily detected insperm from Stra8-iCre; Mov10l1
lox/lox cauda epididymis(Fig. 5d). A semi-quantitative PCR analysis estimatedthat the excision efficiency was �70%. These resultsequivocally demonstrate that sperm carrying unrecom-bined flox allele were indeed present, confirming thepartial Cre-mediated excision in Stra8-iCre; Mov10l1
lox/
lox mice.
CONCLUSIONS
In conclusion, our data demonstrate that Stra8-iCre isnot capable of excising two flox alleles with 100% effi-ciency. In the presence of two flox alleles (lox/lox), thepartial Cre-mediated excision tends to lead to mosai-cism in the targeted cell lineage. However, it can exciseone flox allele with 100% efficiency in pachytene sper-matocytes and spermatids. For this reason, Stra8-iCre;
genelox/D mice represent more of conditional KOs,
whereas Stra8-iCre; genelox/lox mice are more like
mosaic cKOs with a proportion of the target cells retain-ing the flox allele. This may explain why some previous
MOSAICISM BY PARTIAL CRE-MEDIATED EXCISION 5

studies (Kimura et al., 2003; Lei et al., 2010; Rassoulza-degan et al., 2002) report inefficient excision of thefloxed genomic DNA when some germline-specific Cretransgenic lines are used. These and other studies mayhave included data from Cre; gene
lox/lox mice, which
would display much less severe and highly variable phe-notypes compared to Cre; gene
lox/D mice. We believethat this phenomenon is not restricted only to theStra8-iCre line reported here, as we previously foundinsufficiencies of several other germline Cre lines in
FIG. 3. Testicular and epididymal histology of Stra8-iCre; Mov10l11/lox (control), Stra8-iCre; Mov10l1lox/lox (mosaic) and Stra8-iCre;Mov10l1lox/D (true cKO) mice. All testis and epididymis samples were collected from postnatal day 60 males. (a–c) Micrographs of HE-stained testicular cross-sections of mice with the three genotypes. (d) Magnified view of framed area in (c). (e) Diameter of seminiferoustubules in the testes of the three genotypes. In total, 50 round or close to round tubules in the cross-sections as illustrated in the inset werecalculated for statistical analyses (n 5 50, *P<0.01). (f) Percentage of seminiferous tubules displaying a typical spermatogenic stage (a totalof 12 stages in the wild-type mouse) among HE-stained cross-sections derived from mice with the three genotypes (n 5 50). Note that nocross-sections showing any of the 12 typical stages of the seminiferous epithelial cycle are present in Stra8-iCre; Mov10l1lox/D males. (g–i)HE-stained cauda epididymal cross-sections from mice of the three genotypes. Note that mature sperm are completely absent in theStra8-iCre; Mov10l1lox/D epididymis, whereas abundant mature sperm are present in the epididymides with the other two genotypes. Scalebar 5 50 mm.
6 BAO ET AL.

FIG. 4. Spermatogenic disruptions in Stra8-iCre; Mov10lox/lox (mosaic) mice. (a) Normal spermatogenesis in Stra8-iCre; Mov101/lox testesat postnatal day 60. (b) Enlarged image of the framed area in (a), showing spermatogenic stages (marked with Roman numerals) of the sem-iniferous epithelial cycle in Stra8-iCre; Mov101/lox testes at postnatal day 60. All sections were stained using the PAS reagent that allowsaccurate staging based on the shape of the developing acrosome in spermatids. (c–h) Highly variable spermatogenic disruptions in Stra8-iCre; Mov10lox/lox seminiferous tubules. (d–h) Magnified images corresponding to the framed area in (c). (d) Arrows and arrowheads indicateabnormal zygotene spermatocytes and round spermatids, respectively; (e) arrows and arrowheads demonstrate zygotene-like and pachy-tene spermatocytes, respectively; sporadic vacuoles are present, which are generally located in the cytoplasm of Sertoli cells and are indic-ative of active germ cell depletion. (f) A morphologically normal tubule cross-section containing round spermatids (Step 6) (arrows). (g) Aseminiferous tubule contains no elongating/elongated spermatids, suggesting a block in the elongation step of spermiogenesis. Arrowsindicate the arrested Step 4 spermatids. (h) A relatively morphologically normal seminiferous tubule. Scale bar 5 20 mm.
MOSAICISM BY PARTIAL CRE-MEDIATED EXCISION 7

excising two flox alleles (data not shown). Interestingly,the problem of insufficient Cre excision appears to becommon in Cre transgenic lines generated by introduc-ing a short transgenic construct or a large bacterial arti-ficial chromosome fragment containing the Cre geneinto the genome through the classic pronuclear injec-tion method. It is commonly believed that Cre lines gen-erated through targeted insertion of a Cre gene into alocus immediately downstream of the promoter of anendogenous gene, for example Rosa26 locus, tend todisplay better excision efficiency. This notion, however,remains to be validated. To date, it is still unclear whyiCre in the Stra8-iCre line could not efficiently excisetwo flox alleles despite their abundant expression.Nevertheless, this study strongly suggests that it isessential to generate Cre;gene
lox/D mice rather thanCre;gene
lox/lox mice for phenotypic characterizationbecause they are closer to “true” conditional KO mice(excision efficiency, 100%).
MATERIALS AND METHODS
Animals
Stra8-iCre, Rosa26mTmGtg/tg, and Mov10l1
lox/lox
mice were purchased from the Jackson Laboratory (BarHarbor, Maine) and maintained in a temperature- andhumidity-controlled room with free access to food and
water. Animal use was approved by Institutional AnimalUse and Care Committee (IAUCC) of the University ofNevada, Reno. The compound conditional KO mice(Stra8-iCre; Mov10l1
lox/Dor Stra8-iCre; mTmG
1/tg) areavailable to the research community upon request.
Mouse genotyping and sperm DNA extraction
The genotyping primers used were as follows: Stra8-iCre: Forward: GTG CAA GCT GAA CAA CAG GA;Reverse: AGG GAC ACA GCA TTG GAG TC; mov10l1:FW1: TACCCCAGCTGAGAGGTCAC; FW2: CACTGGTGATTCAGGGGACT; Rev: TCCCAGAAGGCCTTACACAC. PCR was performed as described previously with annealingtemperature at 60�C for Stra8-iCre and 56�C forMov10l1, respectively (Frost et al., 2010). For sperm iso-lation, cauda epididymides were dissected and spermwere carefully released into the HTF medium (100mL/per cauda) (Irvine Scientific, Santa Ana, California Cat#:90125) using two sharp tweezers. Sperm DNA extractionwas performed using a commercial kit (IllustraTM
genomicPrep Mini Spin, Cat#: 28-9042-75).
Histological analyses of the testis
Testis paraffin block preparation, HE, and PAS stain-ing were all performed as described previously (Wuet al., 2012).
FIG. 5. Partial Cre-mediated excision in spermatogenic cells of Stra8-iCre; Mov10lox/lox testes as evidenced by the detection of theMov10l1 flox allele in cauda epididymal sperm. (a–c) Phase contract microscopic images of cauda epididymal contents in mice of the threegenotypes indicated (scale bar 5 100 mm). (d) Semi-qPCR analyses of relative levels of the flox and the recombined flox (D) alleles in spermof Stra8-iCre; Mov10lox/lox testes. Two sets of primers were used and the first one (FW1 1 Rev) was used to detect the flox allele, whereasthe other (FW2 1 Rev) was employed for determining the recombined flox (KO, D) allele.
8 BAO ET AL.

Fluorescent imaging
Testicular cryosections were prepared as describedpreviously (Bao et al., 2010) for confocal imaging.Briefly, developing and adult testes were dissected andimmediately fixed in 4% paraformaldehyde solution atroom temperature for 3–5 h with gently shaking, fol-lowed by dehydration with serial sucrose solutions atroom temperature: 10% sucrose for 1 h, 15% for 1 h,and 20% for 3 h. After optimal cutting temperatureembedding, the samples were cut into 10 mm cryosec-tions. Slides were finally counterstained with 40,6-diami-dino-2-phenylindole (DAPI) (1 mg/mL) and images wereacquired using an Olympus FV1000 confocal micro-scope system. Samples were protected from light asmuch as possible during the whole operation processto prevent loss of fluorescence.
ACKNOWLEDGEMENTS
The authors thank the University of Nevada Genetic Engi-neering Center for animal care.
LITERATURE CITED
Anderson EL, Baltus AE, Roepers-Gajadien HL, HassoldTJ, de Rooij DG, van Pelt AM, Page DC. 2008.Stra8 and its inducer, retinoic acid, regulatemeiotic initiation in both spermatogenesis andoogenesis in mice. Proc Natl Acad Sci USA 105:14976–14980.
Aoki K, Taketo MM. 2008. Tissue-specific transgenic,conditional knockout and knock-in mice of genes inthe canonical Wnt signaling pathway. Methods MolBiol 468:307–331.
Bao J, Zhang J, Zheng H, Xu C, Yan W. 2010. UBQLN1interacts with SPEM1 and participates in spermio-genesis. Mol Cell Endocrinol 327:89–97.
Chung SS, Cuzin F, Rassoulzadegan M, Wolgemuth DJ.2004. Primary spermatocyte-specific Cre recombi-nase activity in transgenic mice. Transgenic Res 13:289–294.
Frost RJ, Hamra FK, Richardson JA, Qi X, Bassel-Duby R,Olson EN. 2010. MOV10L1 is necessary for protec-tion of spermatocytes against retrotransposons byPiwi-interacting RNAs. Proc Natl Acad Sci USA 107:11847–11852.
Gallardo T, Shirley L, John GB, Castrillon DH. 2007.Generation of a germ cell-specific mouse transgenicCre line, Vasa-Cre. Genesis 45:413–417.
Gama Sosa MA, De Gasperi R, Elder GA. 2010. Animaltransgenesis: An overview. Brain Struct Funct 214:91–109.
Guan C, Ye C, Yang X, Gao J. 2010. A review of currentlarge-scale mouse knockout efforts. Genesis 48:73–85.
Hammond SS, Matin A. 2009. Tools for the genetic anal-ysis of germ cells. Genesis 47:617–627.
Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, Tang F,Hajkova P, Lao K, O’Carroll D, Das PP, Tarakhovsky A,Miska EA, Surani MA. 2008. MicroRNA biogenesis isrequired for mouse primordial germ cell develop-ment and spermatogenesis. PLoS One 3:e1738.
Hogarth CA, Evanoff R, Snyder E, Kent T, Mitchell D,Small C, Amory JK, Griswold MD. 2011. Suppres-sion of Stra8 expression in the mouse gonad byWIN 18,446. Biol Reprod 84:957–965.
Inselman AL, Nakamura N, Brown PR, Willis WD,Goulding EH, Eddy EM. 2010. Heat shock protein 2promoter drives Cre expression in spermatocytes oftransgenic mice. Genesis 48:114–120.
Kido T, Lau YF. 2005. A Cre gene directed by a humanTSPY promoter is specific for germ cells and neu-rons. Genesis 42:263–275.
Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H,Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T.2003. Conditional loss of PTEN leads to testicularteratoma and enhances embryonic germ cell pro-duction. Development 130:1691–1700.
Kyronlahti A, Euler R, Bielinska M, Schoeller EL, MoleyKH, Toppari J, Heikinheimo M, Wilson DB. 2011.GATA4 regulates Sertoli cell function and fertility inadult male mice. Mol Cell Endocrinol 333:85–95.
Lei Z, Lin J, Li X, Li S, Zhou H, Araki Y, Lan ZJ. 2010. Post-natal male germ-cell expression of cre recombinasein Tex101-iCre transgenic mice. Genesis 48:717–722.
Maatouk DM, Loveland KL, McManus MT, Moore K, HarfeBD. 2008. Dicer1 is required for differentiation of themouse male germline. Biol Reprod 79:696–703.
Miller RL. 2011. Transgenic mice: Beyond the knockout.Am J Physiol Renal Physiol 300:F291–F300.
Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. 2007.A global double-fluorescent Cre reporter mouse.Genesis 45:593–605.
Novak A, Guo C, Yang W, Nagy A, Lobe CG. 2000. Z/EG,a double reporter mouse line that expressesenhanced green fluorescent protein upon Cre-medi-ated excision. Genesis 28:147–155.
Pluck A. 1996. Conditional mutagenesis in mice: TheCre/loxP recombination system. Int J Exp Pathol 77:269–278.
Rasoulpour RJ, Boekelheide K. 2006. The Sycp1-Cretransgenic mouse and male germ cell inhibition ofNF-kappa b. J Androl 27:729–733.
Rassoulzadegan M, Magliano M, Cuzin F. 2002. Transvec-tion effects involving DNA methylation during meio-sis in the mouse. EMBO J 21:440–450.
Sadate-Ngatchou PI, Payne CJ, Dearth AT, Braun RE.2008. Cre recombinase activity specific to postnatal,premeiotic male germ cells in transgenic mice. Gen-esis 46:738–742.
MOSAICISM BY PARTIAL CRE-MEDIATED EXCISION 9

Speck NA, Iruela-Arispe ML. 2009. Conditional Cre/LoxP strategies for the study of hematopoieticstem cell formation. Blood Cells Mol Dis 43:6–11.
Tamowski S, Aston KI, Carrell DT. 2010. The use oftransgenic mouse models in the study of male infer-tility. Syst Biol Reprod Med 56:260–273.
Vidal F, Sage J, Cuzin F, Rassoulzadegan M. 1998. Creexpression in primary spermatocytes: A tool forgenetic engineering of the germ line. Mol ReprodDev 51:274–280.
Wu Q, Song R, Ortogero N, Zheng H, Evanoff R, SmallCL, Griswold MD, Namekawa SH, Royo H, Turner
JM, Yan W. 2012. The RNase III enzyme DROSHA isessential for microRNA production and spermato-genesis. J Biol Chem 287:25173–25190.
Yan W. 2009. Male infertility caused by spermiogenicdefects: Lessons from gene knockouts. Mol CellEndocrinol 306:24–32.
Zheng K, Xiol J, Reuter M, Eckardt S, Leu NA, McLaugh-lin KJ, Stark A, Sachidanandam R, Pillai RS, Wang PJ.2010. Mouse MOV10L1 associates with Piwi pro-teins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc Natl AcadSci USA 107:11841–11846.
10 BAO ET AL.







![Phenotypic and molecular detection of metallo-β-lactamase ... · Akram Azimi[1], Amir Peymani[1] and Parham Kianoush pour[1] [1]. Medical Microbiology Research Center, Qazvin University](https://static.fdocument.org/doc/165x107/5f999ed20fd7b062d8790660/phenotypic-and-molecular-detection-of-metallo-lactamase-akram-azimi1-amir.jpg)