Energy loss and energy straggling a presentation by Younes Sina
-
Upload
younes-sina -
Category
Technology
-
view
1.680 -
download
1
description
Transcript of Energy loss and energy straggling a presentation by Younes Sina

Energy Loss and
Energy Straggling
Younes Sina
The University of Tennessee, Knoxville

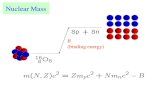
Nt
areal density
dE/dx Stopping power, stopping force, specific energy loss[MeV/mm] , [eV/μm]
ΔE/Δx=dE/dxΔx→0
εstopping cross section[eV/1015 atoms/cm2 ],[ KeV/mg/cm2 ]
ε=1/N(dE/dx)
ε=1/ρ(dE/dx)
[atoms/cm2]
Nvolume density [atoms/cm3]
ρ mass density (gr/cm3)
Nt=N .dx
Basic concepts & definitions

Incident particles Transmitted particles
E0
Z1
M1
E0 -ΔE
Δx
Nt
M2
Z1
Z1
M1
Basic concepts and definitions

Uni
t con
vers
ions
Multiply units by For units Example
MeV MeV/amu 4 MeV 4He ~ 1 MeV/amu
v/vo (MeV/amu) 1/2 v/vo =1~0.025 MeV/amu 1H
(MeV/amu)1/2 m/s 2 MeV 4He ~ vHE =9.82x 106 m/s
1015 atoms/cm2 nm 1018 Atoms/cm2 For Au~170nm
μg/cm2 nm 100 μg/cm2 For C~258 nm
μg/cm2 1015 atoms/cm2 100 μg/cm2
For Au~305x1015 atoms/cm2
eV cm2/1015 atoms MeV/(mg/cm2 ) 100 eV cm2/1015 atoms for Al2O3~2.95 MeV cm2/mg
[M2= (2MAl + 3 MO)/5; MAl=26.98, MO=16.00]
eV cm2/1015 atoms keV/μm 30eV cm2/1015 atoms for Si~150 keV/μm
][1
1
amuM
]3/[
][10661.1 22
cmg
amuM
]/[
103cmg
][661.110
1
3
amuM
1581.0
10389.1 7
][661.11
2 amuM
][661.1
]/[102
32
amuMcmg

Basic Physics
Important factors during interaction of ions and target:
Ion velocityCharge of the ionCharge of target atom

Energy loss of ions:3 regimes for ions:
Low velocityIntermediate velocityHigh velocity
In comparison to the orbital velocity of atomic electron

V (velocity of ions)<<< v0 (velocity of electron at orbital)
V0= Bohr velocity
elastic collision with target nuclei
Nuclear energy loss dominates
nuclear energy loss diminishes as 1/E
Electronic energy loss dominates (inelastic collisions with atomic electrons)
Low velocityIntermediate velocityHigh velocity
ΔE= ΔEn+ΔEe
The ion carries its electrons and tends to neutralize by electron capture
With increasing v

v≈ 0.1v0 to v≈Z12/3v0 ΔEe
v>>v0 : charge state of the ion increases ion becomes fully stripped of its electrons
In the low –ion-velocity range
E
In the high –ion-velocity range
v>>v0 ΔEe chargeion

dE/dx=N .Z2 .(Z1e2)2 f (E/M1) (E/M1) is a function of target (it is not a function of the projectile)
for most application of ion beam analysis, nuclear stopping is small. Above 200 keV/amu contribution of nuclear stopping <1%
For example for Zr (amu=90): at E≥2.22 keV contribution of nuclear stopping <1%
Bethe & Bloch formula for high- velocity regime

EHI= (mHI/mH)EH≈ mHIEH
The scaling rule
γ= fraction effective charge
higher energy of ion γ→1
εHI = εHγHI 2ZHI
2
ε = 1/N(dE/dx)

EH=EHe/mHe→EH =2MeV/4=0.5 MeV
Example
If γHe=1 EHe=2 MeVWhat is EH ?
EHI= (mHI/mH)EH≈ mHIEH

Example 2:
If γLi=1 Calculate εLi@2,5,and 10 MeV
EHI=mHIEHEH=ELi/mLi
EH=ELi/7EH=2000/7=285 keVEH=5000/7=714.28 keVEH=10000/7=1428.57 keV
εH@285 keV= 0.489εH@714 keV= 0.282εH@1428 keV= 0.177
εLi=9εH
εHI =εHγHI 2ZHI
2 εLi =εHγLi 2ZLi
2
εLi=4.40 Mev.cm2/mgεLi=2.54 Mev.cm2/mgεLi=1.60 Mev.cm2/mg

Effective charge (γ) as a function of Z1 &Z2
ai : fitting constant value
E/M1 [keV/amu]
M1 = 4.0026
a0=0.2865a1=0.1266a2=-0.001429a3=0.02402a4=-0.01135a5=0.001445
If E He=0.5 MeV :γHe
2ZHe2 = (γHe
2). (22)=2.88
If E He=1 MeV :γHe
2ZHe2 = (γHe
2). (22)=3.46
If E He=1.5 MeV :γHe
2ZHe2 = (γHe
2). (22)=3.75
If E He=2 MeV :γHe
2ZHe2 = (γHe
2). (22)=3.89
If E He=3 MeV :γHe
2ZHe2 = (γHe
2). (22)=3.99
If γHe=1 then:γHe
2ZHe2 = (12). (22)=4.00
1
5
0
exp1 )][ln(2
M
Ea
i
iiHe

γLi =A1-exp[-(B+C)]
Effective charge (γ) as a function of Z1 &Z2
A=1+ (0.007+5x10-5Z2)exp -[7.6-ln(ELi[keV/amu]2
B=0.7138+0.002797ELi[keV/amu]
C=1.348x10-6 (ELi[keV/amu]2)

Calculation of γLi ,stopping in carbon at 2,5 and 10 MeV
mLi=7 ELi= 2, 5, and 10 Mev
ELi/mLi = ELi/7= 2857151430
γLi =A1-exp[-(B+C)]A=1+ (0.007+5x10-5Z2)exp -[7.6-ln(ELi[keV/amu]2
B=0.7138+0.002797ELi [keV/amu]
C=1.348x10-6 (ELi[keV/amu]2)
γLi =0.80.971
εLi =εHγLi 2ZLi
2
εLi =εHγLi 2(3)2
εLi =9εHγLi 2 εLi = 2.81
εLi = 2.38εLi = 1.593
εLi =9εHγLi 2
εLi =9εHγLi 2
εLi =9εHγLi 2
From Example 2:εH@285 keV= 0.489εH@714 keV= 0.282εH@1428 keV= 0.177

Effective charge (γ) for heavy ions : Z > 3
γHI =1- exp (-A)[1.034-0.1777 exp(-0.08114 ZHI)]
A=B+0.0378 sin (π B/2)
B=0.1772 (EHI [keV/amu]) 1/2 ZHI -2/3

Bragg’s rule
Stopping cross section for compound
εAB =mεA+nεB
Example : 2.0 MeV ion 4He stopping in silicon SiO2
SRIM-2006 gives εSi(2.0 MeV)=46.88 eV cm2/1015 atoms and εO(2.0 MeV)=38.36 cm2/1015 atoms. For , SiO2 we then have εSiO2 =1εSi+2εO =41.02 cm2/1015
atoms

Stopping cross section and depth scale
ΔE=
x
dxdxdE0
)/(
X= 0
)/(
1E
E
dEdxdE
dE/dx Stopping power
εstopping cross section
ε=(1/N)(dE/dx)
εCan be evaluated either at E0 or at Eav=E0-ΔE/2

Thin targets
x
dxdxdE0
)/(ΔE=
ΔE= (dE/dx) (E0)
Δx
ΔE=ε(E0)Nt
ε=(1/N)(dE/dx)
ΔE=ε(Eav)Nt
ΔE= (dE/dx) (av)
Δx
Surface energy approximation Mean energy approximation

Thick targets
ΔEi= (dE/dx) (Ei-1)
Δxi
ΔEi=ε(Ei-1)(Nt)i
n
iiEE
1
E0
Energy loss evaluated at the energy of the ion at the ( i-1)
the slabStopping cross section evaluated at the energy of the ion at the ( i-1) the slab

Example: Proton depth scale in carbon
What is the 2.0 MeV proton energy lost in a carbon target for depth of (a) 1000 nm and (b) 20 μm?
From the unit conversion table:
1015 atoms/cm2=]3/[
][10661.1 22
cmg
amuM
nm
1000 nm= 17.6x 1018 atoms/cm2
20 μm= 353x 1018 atoms/cm2
ΔE=ε(E0)Nt
ε(E0=2MeV)=2.866 ev cm2/1015 atomsΔE=2.866x10-15x17.6x1018≈50 keV
ΔE=ε(E0)Nt
ε(E0=2MeV)=2.866 ev cm2/1015 atomsΔE=2.866x10-15x353x1018≈1000 keV
Surface energy approximation Thin targets

Example: Proton depth scale in carbon
What is the 2.0 MeV proton energy lost in a carbon target for depth of (a) 1000 nm and (b) 20 μm?
From the unit conversion table:
1015 atoms/cm2=]3/[
][10661.1 22
cmg
amuM
nm
1000 nm= 17.6x 1018 atoms/cm2
20 μm= 353x 1018 atoms/cm2
ΔE=ε(Eav)Nt Eav=E0-ΔE/2=2000-50/2 keV=1975 keVε(Eav=1975 keV)≈2.866 ev cm2/1015 atomsΔE=2.866x10-15x17.6x1018≈50 keV
ΔE=ε(Eav)Nt Eav=E0-ΔE/2=2000-1000/2 keV=1500 keVε(Eav=1500 keV)=3.506 ev cm2/1015 atomsΔE= 3.506 x10-15x353x1018≈1235 keV
Mean energy approximation Thin targets

Example: Proton depth scale in carbon
What is the 2.0 MeV proton energy lost in a carbon target for depth of (a) 1000 nm and (b) 20 μm?
Thick targets
ΔEi=ε(Ei-1)(Nt)i
i=6(Nt)i =(353/6)x1018 =58.83x1018 atoms/cm2
ΔE1= ε(E0)(Nt)1= 2.866x10-15x58.83x1018≈168.5 keVThe energy at the end of the first slab is then E1=E0-ΔE=2000-168.5 keV=1832 keVEnergy loss in the second slab at this energy: ΔE2= ε(E1)(Nt)2=3.051x10-15x58.83x1018≈179.5 keVE2=E1-ΔE2=1832-179.5keV=1652 keV …….E3= 193.0 , E4= 210.3 , E5=233.7 , E6= 268.1 keV
ΔE2=Σ ΔEi(i=1-6)=1253 keV
E0E1E2

electronic stopping for isotopes
Stopping (medium [ ,Z2]) =stopping (medium [Mav , Z2]).(Mav/ )M av
M av

Straggling
Nt[atoms/cm2]≥2x1020. 2Z
1)
Z]/[
(1
2
amuMeVE
Bohr’s theory:
When the energy transferred to target electrons in the individual collisions is small compare to the width of the energy loss distribution, the distribution is close to a Gaussian distribution.
In the limit of high ion velocity, the energy loss is dominated by electronic excitations.
ΩB2[keV2]=0.26Z1
2Z2Nt[1018 atoms/cm2]
Full width at half- maximum height(FWHM)=2.355Ω
Bohr value for the variance (standard deviation) of the average energy loss fluctuation

Example
ΩB2[keV2]=0.26Z1
2Z2Nt[1018 atoms/cm2]
From the following Equation, we obtain for 4He ions:
ΩB2[keV2]≈Z2Nt[1018 atoms/cm2]
Helpful for quick estimates of 4He ion Bohr straggling4% accuracy

Corrections to Bohr’s theory, other models
Ω2/ΩB2= 0.5 L(x), for E [keV/amu]< 75 Z2
1, for E [keV/amu] ≥ 75 Z2
L(x)=1.36 x1/2- 0.16 x3/2
225
]/[
Z
amukeVEx
Lindhard & Scharff Eq.:

Example
Straggling of 5.0 MeV helium ions in gold
From Bohr Eq.: ΩB2[keV2]=0.26Z1
2Z2Nt[1018 atoms/cm2]
we have: ΩB
2/Nt≈ 82 keV2 cm2/1018 atoms In a gold layer of 1018 atoms/cm2 (about 170 nm) ΩB≈ 9 keV Ω2/ΩB
2≈ 0.8 for He ions in gold at 5.0 MeV Ω=7 keV

Straggling in mixtures and compounds
For an compound (mixture) AmBn (m+n=1) with an atom density NAB[atoms/cm3]and the atomic densities NA and NB:
If mNAB=NA and nNAB=NB then:
(ΩAB)2=(ΩA)2+(ΩB)2
t is the thickness
tB
Bn
tA
Am
t NNNAB
AB
)))(222
((

Example
AmBn = SiO2
Bohr straggling of 4He ions in 1018 atoms/cm2 of SiO2
m=0.33 & n=0.67
Nsi t = 0.33NSiO2t = 0.33x1018 atoms/cm2
NO t = 0.33NSiO2t = 0.67x1018 atoms/cm2
ΩB2[keV2]=0.26Z1
2Z2Nt[1018 atoms/cm2] Bohr’s Eq.
(ΩBSi)2[keV2]=0.26x 0.33Z1
2Z2 = 4.80 keV2
(ΩBO)2[keV2]=0.26x 0.67Z1
2Z2 = 5.57 keV2
(ΩBSiO2)2=(ΩB
Si)2+(ΩBO)2
(ΩBSiO2)2=(4.80+5.57)keV2= 3.22 keV

Additivity of energy loss fluctuations
(ΩTOT)2=(ΩDET)2+(ΩSTR)2 +(ΩBEAM)2
Beam energy profile
Energy resolution
Energy straggling

Range
E
ESESN
dEER
ne0 )]()([)(

Kurdistan, Iran