d ( ) ) =−1 ( coupled ) - MIT - Massachusetts Institute...
Click here to load reader
Transcript of d ( ) ) =−1 ( coupled ) - MIT - Massachusetts Institute...

Hydrostatic Pressure in Liquids
3specific weight Ngm
γ ρ = = =
82 45.67 10 W
m Kσ − = ⋅
Power= Force * Velocity = VI
( )2 1 2 1p p z zγ− = − −
F pdA= ∫
updownp p zγ= + ∆
Any two points at the same elevation in a continuous mass of same static fluid will be at the same pressure. Accelerating liquids
1tan angle between horiz and surfacex
z
ag a
θ −= =+
Buoyancy
Volume Displaced BF γ= ⋅
1
immersed
BB B Bimmersed V
x x x xdVV′ ′= − = ∫
tanBB O
submurged
x IMB MG GB
Vθ′= = = +
stable if 0MG >
2 2
original waterlineO
waterline
I x dA x dA= ≈∫ ∫
Reynolds transport theorem
velocity, mass, outward normalrelv M n= = =r r
( )CMrel
CV CS
dB dbdV b v n dA
dt dtρ ρ= + ⋅∫ ∫
r r Bb M=
Conservation of mass
( )relCV CS
ddV v n dA
dtρ ρ= − ⋅∫ ∫
r r
CVin out
in out
dMm m
dt= −∑ ∑& &
( )cv cs
system
dmdA
t dtρ
ρ∂ + = ∂ ∫ ∫ V ng
Linear Momentum
unit vector, normal vector, outward positiveun n= =r r
( ) ( )
( )
uCV CS CS
relCV CS
dvdV Pn dA dA
dt
gdV v v n dA
ρ τ
ρ ρ
= − + +
− ⋅
∫ ∫ ∫
∫ ∫
r r r
r r r r
( ) ( )
( ) ( )
rel uCV CS CS
rel relin outin outCV
dv dV Pn dA dA
dt
gdV mv mv
ρ τ
ρ
= − + +
+ −
∫ ∫ ∫
∑ ∑∫
r r r
r r r& &
( )rel relCV CS
dF v dV v v n dA
dtρ ρ= + ⋅∑ ∫ ∫
r r r r
Bernoulli Equation Important to approximate the unsteady term.
absolute gauge atmP P P= +
( ) ( )2 2 2 2
2 1 2 11 1
1 10
2v
ds dp v v g z zt ρ
∂+ + − + − =
∂∫ ∫r
r r
( ) ( )2 2
2 12 12 1 0
2
v vp pg z z
ρ
−−+ + − =
r r
Angular Momentum
( ) ( ) ( )
( ) ( )
rel surfaceCV CV
shaft rel relCS
d r v dV r F r g dVdt
T r v v n dA
ρ ρ
ρ
× = × + × +
− × ⋅
∑∫ ∫
∫
rr r r r r
r r r r r
( ) ( ) ( )
( ) ( )
rel surface shaftCV CV
in rel out relin outin out
dr v dV r F r g dV T
dt
m r v m r v
ρ ρ
× = × + × + +
× − ×
∑∫ ∫
∑ ∑
r rr r r r r
r r r r& &
( )( ) ( ) ( )o rel rel relcv cs
dv dV v v dA
dtρ ρ= × + ×∑ ∫ ∫M r r n
r r rg
Pipe Flows
eRvd vdρµ ν
= =r r
pipe diameterd =
µν
ρ=
2300tran =
2 21 21 1 1 2 2 2
1 12 2 f
p pv gz v gz ghα α
ρ ρ+ + = + + +
r r
2
2fL v
h fd g
=r
2
8 wfvτ
ρ= r
2
2fL v p
h f zd g gρ
∆= = ∆ +
r
2
, 4
64 1282f lam
L v LQh
vd d g gdµ µ
ρ πρ= =
rr
1.111 6.91.8log
Re 3.7d
df
ε ≈ − +

( )3 2
2
ReRe
2f d
d
gd h ffcn
Lξ
ν= = =
1.775Re 8 log
3.7d
dεξ
ξ
= − +
Q udA= ∫ First Law
( )2 1 0iso
E E− = ( )2 1 1 2 1 2E E Q W− −− = −
( )uncoupled sys (coupled) kin ela grav ther coupledE E E E U U= + + + +
W F drδ− = ⋅ 2
1
1 2
r
r
W F dr−− = ⋅∫
1 2 1 2
dUQ W
dt− −− =& &
Constitutive Relations
212kinE mv=
dvF ma m
dt= =
2
1
1 2
v
v
W mvdv−− = ∫
212elaE kx= F kx=
graE mgh= F mg=
1 1theU CT mcT= = 22 1
1
lnT
S S mcT
− =
PV mRT= 1revW PdVδ = 2
1
1 2 1
V
V
W PdV− = ∫
gas vU mc T= p vc c R= + p
v
c
cγ = constantPV γ =
vMa
RTγ=
r
2 22 1
1 1
ln lnp
T PS S mc mR
T P
− = −
2 22 1
1 1
ln lnv
T VS S mc mR
T V
− = +
2 22 1
1 1
ln lnv p
P VS S mc mc
P V
− = +
Reversible adiabatic
11
2 21 2 1 1
1 1
1 1v vP V
W m c T mc TP V
γγ
γ−
−
−
− = − = −
Second Law
( )2 1 0 genisoS S S− ≥ =
2
1
A
A
ABtrans
A
QS
Tδ
= ∫
( )2
2 11
A
A
genA
QS S S
Tδ
− = +∫
Flow Field
2 2 2
2 2 2x
p u u u dug
x x y z dtρ µ ρ
∂ ∂ ∂ ∂− + + + = ∂ ∂ ∂ ∂
2 2 2
2 2 2y
p v v v dvg
y x y z dtρ µ ρ
∂ ∂ ∂ ∂− + + + = ∂ ∂ ∂ ∂
2 2 2
2 2 2z
p w w w dwg
z x y z dtρ µ ρ
∂ ∂ ∂ ∂− + + + = ∂ ∂ ∂ ∂
xyu vy x
τ µ ∂ ∂
= + ∂ ∂ xz
w ux z
τ µ∂ ∂ = + ∂ ∂
zyv wz y
τ µ ∂ ∂
= + ∂ ∂
( )0
x
shear wF b x dxτ= ∫
Heat Transfer Conduction
c
dTQ kA
dx = −
& W
kmK
= ( )1 2
ckAQ T T
L= −&
Convection
( )c s sQ h A T T∞= −&
Radiation
( )1 2r sQ h A T T= −& 314r mh Tε σ= 1 2
2m
T TT
+ =
Thermal Resistance
1 2
total
T TQ
R −
=
& convection in parallel with radiation
cond
LR
kA=
1conv
c
Rh A
= 1
radr
Rh A
=
,
ln
2
o
icondradial
RR
RkLπ
=
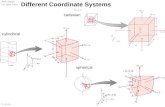
Heat Diffusion Equation alpha = thermal diffusivity Cartesian

genq =& rate of energy dissipated per unit mass by all energy storage
methods other than thermal. q =& rate of energy generated ie
genq
qρ
=&
&
2 2 2
2 2 2
T k T T T qt c x y z cρ ρ
∂ ∂ ∂ ∂= + + + ∂ ∂ ∂ ∂
&
kc
αρ
=
Cylindrical
2
1 1p
T T T Tc kr k k q
t r r r r z zρ
φ φ ∂ ∂ ∂ ∂ ∂ ∂ ∂ = + + + ∂ ∂ ∂ ∂ ∂ ∂ ∂
&
Spherical
22 2 2 2
1 1 1sin
sin sinpT T T T
c kr k k qt r r r r r
ρ θθ φ φ θ θ θ
∂ ∂ ∂ ∂ ∂ ∂ ∂ = + + + ∂ ∂ ∂ ∂ ∂ ∂ ∂ &
Entropy Generation Equation 22 2
2gen
gen
qk T T Ts
T x y z Tρ
∂ ∂ ∂ = + + + ∂ ∂ ∂
&&
gen genS s dxdydzρ=& & s=per unit mass
Fin Temperature Distributions
T Tθ ∞≡ − ( )0b bT Tθ θ ∞= = − 2
c
hPm
kA≡
c bM hPkA θ≡
Convection ( )x L
dh L k
dxθ
θ=
= −
( )( )
cosh ( ) sinh ( )
cosh sinhb
hm L x m L xmkhmL mLmk
θθ
− + −=
+
( )( )
sinh cosh
cosh sinhf
hmL mLmkq MhmL mLmk
+=
+
Adiabatic
0x L
ddxθ
=
= cosh ( )
coshb
m L xmL
θθ
−= tanhfq M mL=
Prescribed Temperature
( ) LLθ θ=
sinh sinh ( )
sinh
L
b
b
mx m L x
mL
θθθ
θ
+ − =
cosh
sinh
L
bf
mLq M
mL
θθ−
=
Infinite fin
( ) 0Lθ = mx
b
eθθ
−= fq M=
Fin Efficiency
max
f ff
f b
q qq hA
ηθ
= =
Total array efficiency
exposed surfacetotal finA NA A= + max c total bQ h A θ=&
( )1 1finsurface fin
total
NA
Aη η= − − maxtotal surfaceQ Qη=& &
Biot Number
cs
VL A=
internal conduction resistance1external convection resistance
solid c c c
solidc s
Lk A h L
Bik
h A= = ≈
1 2 1 2
dUQ W
dt− −− =& &
1Bi = Lumped thermal capacitance model Reversible in terms of the solid. Principal resistance to heat transfer lies within the fluid. Temperature of solid can be modeled as uniform at all times even though it is changing in time.
kc
αρ
= ( )1th th
c s c s
cVcV R C
h A h Aρ
τ ρ
= = =
c sh At
BiFocV
i
e eρθθ
−− ⋅= = 2
c c
t tFo
L tα
= = tc is time for
disturbance to diffuse characteristic length
2 2c s c c c solid c c
c solid c solid c
hAt ht hL k hLt tBi Fo
cV cL k c L k Lα
ρ ρ ρ
= = = = ⋅
( )c s
dTcV h A T T
dtρ ∞= − − ( ) 0gen solid
S =
( ) ( ) ( )2
ln 12
solidgen solidfluid
cV T T TS cV
T T T
ρρ
∞ ∞ ∞
∆ ∆ ∆= = − +
( )0 0
( ) lnfinal finalt t
fc strans solid
i
Th A T TQS dt dt cV
T T Tρ∞
=
− = =
∫ ∫
&
Bi → ∞ Fluid behaves as heat reservoir. Principal resistance to heat transfer lies within the solid. Temperature of fluid can be modeled as uniform at all times even though it is changing in time.
( )2
212
0
2 1 1cos
1 22
nn Fo
nb
xe n
Ln
πθπ
θ π
∞ − +
=
− = + +
∑

2
22 ( ) .2
Fosolid i
c
k T TQe Fo
A L
π − ∞ −= ≥
&
( ) .05solid i
c
k T TQFo
A tπα∞−
= ≤&
Semi Infinite Model
2
2
T Tt x
α∂ ∂
=∂ ∂
with boundary conditions
i
s i
T TT T
θ−
=−
4x
tη
α=
2
0
2 1uerf e du erfc erf
η
η η ηπ
−= = −∫
Case I Constant Surface temperature
i
s i
T Terfc
T Tθ η
−= =
−
( )solid s i
c
k T TQA tπα
−=
&
Case II Constant Heat Flux at Surface
2
444
xt
iQ t x
T T e xerfcAk t
ααπ α
− − = −
&
Case III Convective Heat Transfer at the Surface
2c ch x h
tk ki c
s i
T T herfc e erfc t
T T k
α
η η α
+ − = − + −
Case IV Surface Energy Pulse
2
4x
ti
ET T e
c tα
ρ πα
−− =
Case V Periodic Variation of Surface Temperature
2
2xi
s i
T Te sin t x
T Tω α ω
ωα
− −= −
−
Two Semi -Infinite Solids in Simple Thermal Communication
( ) ( )( ) ( )
, ,A i B iA Bs
A B
T k c T k cT
k c k c
ρ ρ
ρ ρ
+=
+
Reversible Cycles Carnot Cycle
0H Ltransfer
H L
Q QQS
T T Tδ
δ = = + =∫ ∫Ñ Ñ
( ) 1H LH L H
H H
Q TW T T Q
T Tδ
= − = −
∫Ñ
H
W
Q
δη = ∫Ñ
11
LH
H Lrev
H H
TQT T
Q Tη
−
= = −
1
1
L
H
L
QCOP
QWQ
δ= =
− − −∫Ñ
1
1rev
H
L
COPTT
=−
H HHP
H L
Q QCOP
Q QWδ= =
+∫Ñ ( ) 1
1HP rev
L
H
COPTT
=−
The energy transferred in the form of a heat transfer from a higher temperature source has a higher “quality” (greater value for energy conversion purposes) than the energy transferred as a heat transfer from a lower temperature source because it carries less entropy.
0H Ltransfer gen gen
H L
Q QS S S
T Tδ δ δ+ = + + =∫ ∫ ∫Ñ Ñ Ñ
( ) ( ) L genrev irrevW W T Sδ δ δ− =∫ ∫ ∫Ñ Ñ Ñ
Lirrev rev gen
H
TS
Qη η δ= − ∫Ñ
1
1irrev
H Hgen
L L
COPT T
ST Q
δ=
− + ∫Ñ
Temperature Distributions Plane Wall (x=0 at middle of wall, x= +L or –L at ends of wall, -L=T1
2 2,2 ,1 ,1 ,2
2( ) 12 2 2
s s s sT T T TqL x xT x
k L L− +
= − + +
&
( ) ( ),2 ,12 s s
kq x qx T T
L′′ = − −&
( ) ( ),2 ,12 s s x
kq x qx T T A
L = − −
&
Cylindrical Wall
( ) ( )( )
2 2 2222 2 1
,2 ,2 ,12 22 2 2 1
ln( ) 1 1
4 4 lns s s
r rqr qr rrT r T T T
k r k r r r
= + − − − + −
& &
( )
( )
2 22 1
,2 ,122
2 1
14
( )2 ln
s sqr rk T T
k rqrq rr r r
− + −
′′ = −
&&
( )2 1ln2cond
r rR
Lkπ=
Spherical Wall
( )2 2 22
2 2 1 2,2 ,2 ,12 2
2 2 1 2
1 1( ) 1 6 1
6 4 1 1s s s
qr qr r r rrT r T T T
k r k r r r
−= + − − − + − −
& &

( )
[ ]
2 22 1
,2 ,122
21 2
16
( )3 1 1
s sqr rk T T
k rqrq rr r r
− + −
′′ = −−
&&
1 2
1 1 14condR
k r rπ
= −
Summary of Governing Relations for a Control Volume
t h e r m a l e n e r g yu = 1v= = s p e c i f i c v o l u m eρ
J
h u Pvkg
= +
H U PV mh= + =
Reynolds Transport Theorem
( )CM
CV CS
dB dbdV b n dA
dt dtρ ρ ϑ= + ⋅∫ ∫
r r Bb M=
Conservation of Mass (Continuity Equation)
( )CV CS
ddV n dA
dtρ ρ ϑ= − ⋅∫ ∫
r r CV
in outin out
dMm m
dt= −∑ ∑& &
First Law of Thermodynamics
( )2 2
2 2shaft rCV CS
d v vh gz dV Q W h gz v n dAdt
ρ ρ + + = − − + + ⋅ ∫ ∫
r r& &
2 2
2 2CV
shaft in outin outin out
dE v vQ W m h gz m h gz
dt
= − + + + − + +
∑ ∑& & & &
Second Law of Thermodynamics
( ) geniCV CSi
d QsdV s n dA S
dt Tρ ρ ϑ
= − ⋅ +
∑∫ ∫
& r r &
( ) ( )CVgenin out
i in outi
dS Qms ms S
dt T
= + − +
∑ ∑ ∑&
&& &
Linear Momentum Equation
( ) ( ) ( )rCV CS CS CV CS
d dV Pn dA dA gdV n dAdt
ρϑ ρ τ ρ ρϑ ϑ= − + + + ⋅∫ ∫ ∫ ∫ ∫r r rr r r r
( ) ( ) ( ) ( )in out
in outCV CS CS CV
d dV Pn dA dA gdV m mdt
ρϑ ρ τ ρ ϑ ϑ= − + + + −∑ ∑∫ ∫ ∫ ∫r r rr r r & &
Angular Momentum Equation
( ) ( ) ( ) ( ) ( )surface shaft rCV CV CS
d r dV r F r g dV T r n dAdt
ϑ ρ ρ ϑ ρ ϑ
× = × + × + − × ⋅
∑∫ ∫ ∫r r rr rr r r r r r
( ) ( ) ( ) ( ) ( )surface shaft in outin outin outCV CV
d r dV r F r g dV T m r m rdt
ϑ ρ ρ ϑ ϑ
× = × + × + + × − ×
∑ ∑ ∑∫ ∫r r rr rr r r r r r& &
general coordinate information dA rdrdθ= quasi static= passing through a series of equilibrium states.
Accelerationm
s2 Area m2
Densitykg
m3 Energy J
J 1 kg m2 s 2= Force N N 1 kg m s 2= HeatTransferRate W W 1 kg m2 s 3= Heatflux
W
m2
HeatGenRateW
m3 HeatTransferCoeff
W
m2 K.
KViscositym2
s LatentHeat
J
kg
J
kg1 m2 s 2=
Length m
Mass kg MassDensitykg
m3
MassFlowRatekg
s MassTransferCoef
1
m
Power W W 1 kg m2 s 3= PressureStress
N
m2 SpecificHeat
J
kg K.
Temperature K TempDiff K ThermalCond
W
m K. W
m K.1 kg m s 3 K 1=
ThermalResistK
W K
W1 kg 1 m 2 s3 K=
DViscosityN s.
m2 Volume m3
VolumeFlowRatem3
s


Definitions and such When to use Bernoulli.
1. Steady flow. (otherwise, use unsteady version and estimate unsteady term.
2. Incompressible flow- Mach number less than .3. 3. Frictionless flow

4. Flow along a single streamline 5. No shaft work-no pumps or turbines. 6. No heat transfer, ie adiabatic.
Heat Transfer The energy transfer interaction that occurs between pure thermal systems Temperature The property of a system which indicates the potential for heat transfer with other systems. Two systems are unequal in temperature when they experience heat transfer. For a heat transfer in the absence of work transfer, the heat transfer is positive for the low temperature system which increases in energy and negative for the high temperature systems which decreases in energy A quasi -static process is a model for a dynamic process in which the state of the system is changing at a rate which is slow compared to the rate at which the system approaches the equilibrium state by means of energy and entropy transfer processes internal to the system boundary. Internally, the system appears to be in equilibrium at all times throughout the process even though its state is changing with time. The equilibrium properties thus provide a complete description of the state of the system at all times throughout the process. A reversible process generates no entropy, is a sequence of equilibrium states, and proceeds in the reverse direction just as readily as in the forward direction and takes an infinitely long time to be carried out. Head loss is the change in the sum of the pressure and gravity head, the change in height of the hydraulic grade line or height change of the energy grade line System: A system is defined as an quantity of matter or region of space to which attention is directed for purpose of analysis. Boundary: The quantity of matter or region of space which forms the system is delineated by a boundary, a surface either real or imaginary. State and Property: State is used to signify the condition of a system at a specific instant. The state of a system is characterized by a collection of observable, macroscopic quantities, called properties. A property is one of those observable macroscopic quantities which are definable at a particular instant without reference to the system’s history.









![ft - ΕΚΠΑ - Προσωπικές Ιστοσελίδεςusers.uoa.gr/~nchilak/vivlio/Parts/17 P.pdf · Cartesian parabola, Kapt&cnavi] ... parabolic branch (of a curve), na ...](https://static.fdocument.org/doc/165x107/5ab961647f8b9ad13d8d9e0a/ft-usersuoagrnchilakvivlioparts17.jpg)