Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in...
Transcript of Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in...

Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
Contents lists available at ScienceDirect
Journal of Molecular and Cellular Cardiology
j ourna l homepage: www.e lsev ie r .com/ locate /y jmcc
Original article
Curcumin protects hearts from FFA-induced injury by activatingNrf2 andinactivating NF-κB both in vitro and in vivo
Chunlai Zeng a,b,1, Peng Zhong a,b,1, Yunjie Zhao b, Karvannan Kanchana b, Yali Zhang b, Zia A. Khan c,Subrata Chakrabarti c, Lianpin Wu d, Jingying Wang b, Guang Liang b,⁎a Department of Cardiology, The 5th Affiliated Hospital, Wenzhou Medical University, Lishui, Zhejiang, Chinab Chemical Biology Research Center, School of Pharmaceutical Science, Wenzhou Medical University, Wenzhou, Zhejiang, Chinac Department of Pathology, Western University, London, ON N6A5C1, Canadad Department of Cardiology, The 2th Affiliated Hospital, Wenzhou Medical University, Wenzhou, Zhejiang, China
⁎ Correspondence to: G. Liang, Chemical BiologyPharmaceutical Sciences, Wenzhou Medical UniversTel./fax: +86 577 86699396.
E-mail address: [email protected] (G. Liang)1 These authors contribute equally to this work.
http://dx.doi.org/10.1016/j.yjmcc.2014.10.0020022-2828/© 2014 Elsevier Ltd. All rights reserved.
a b s t r a c t
a r t i c l e i n f oArticle history:Received 19 September 2014Accepted 7 October 2014Available online 16 October 2014
Keywords:Free fatty acidsCardioprotectionCurcuminOxidative stressInflammation
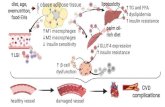
Obesity and increased free fatty acid (FFA) level are tightly linked, leading to the development of cardiovasculardisorders. Curcumin is a natural product from Curcuma longa with multiple bioactivities and is known to havecardioprotective effects in several cellular and animal models. The current study was designed to evaluate thecardioprotective effects of curcumin and demonstrate the underlying mechanism in FFA-induced cardiac injury.Using cell culture studies and high fat in vivomodel, we explored themechanistic basis of anti-inflammatory andantioxidant activities of curcumin. We observed that palmitate (PA) treatment in cardiac derived H9C2 cellsinduced a marked increase in reactive oxygen species, inflammation, apoptosis and hypertrophy. All of thesechanges were effectively suppressed by curcumin treatment. In addition, oral administration of curcumin at50 mg/kg completely suppressed high fat diet-induced oxidative stress, inflammation, apoptosis, fibrosis, hyper-trophy and tissue remodeling inmice. The beneficial actions of curcumin are closely associatedwith its ability toincrease Nrf2 expression and inhibit NF-κB activation. Thus, both in vitro and in vivo studies showed a promisingrole of curcumin as a cardioprotective agent against palmitate and high fat dietmediated cardiac dysfunction.Weindicated the regulatory roles of Nrf2 and NF-κB in obesity-induced heart injury, and suggested that theymay beimportant therapeutic targets in the treatment of obesity-related disorders.
© 2014 Elsevier Ltd. All rights reserved.
1. Introduction
The prevalence of obesity has increased dramatically worldwideover the last decades. Obesity is characterized by a chronic inflammato-ry state that plays a crucial role in obesity-related pathologies such ascardiovascular diseases, type 2 diabetes, and even cancer [1–3]. Chronicaccumulation of excess body fat leads to a variety of metabolic changes.In addition, hyperlipidemia activates systems modulating oxidativestress and inflammation, increasing the prevalence of cardiovasculardisease risk factors [4]. It is well known that obesity is associated withelevated circulating free fatty acid (FFA) levels including palmitate(PA) which cause chronic inflammation, insulin resistance, and car-diovascular disease [5]. These processes are collectively termed
Research Center, School ofity, Wenzhou 325035, China.
.
“lipotoxicity” which occur in many peripheral tissues such as theliver, heart, skeletal muscle and pancreas. There have been severalreports describing the direct effect of a high fat diet (HFD) and itsrelated metabolite such as FFAs and ceramides, on cardiovasculardysfunction [6–8].
FFAs can increase the expression of proinflammatory cytokines andinduce cellular oxidative stress. It has been shown in vivo and in vitrothat FFAs can activate the canonical proinflammatory nuclear factor-κB (NF-κB) pathway. Acute increases in plasma FFA levels activatedNF-κB in human skeletal muscle [9], therefore increasing expression ofseveral proinflammatory cytokines including tumor necrosis factor-α(TNF-α), interleukins (IL) 1-β and -6 in the rat liver as well as an in-crease in circulating monocyte chemotactic protein-1 (MCP-1) levels[10]. PA-induced apoptosis has been well demonstrated in a numberof culture studies [11–13]. These studies have also reported that in-creased FFAsmay be active in the cellular oxidation–reduction reactionsresulting in the formation of excess lipid-derived free radicals [14,15].The concept that emerges from these observations is that elevated plas-ma FFA levels, either as a result of obesity or a high fat diet, can producea combined state of low grade inflammation and oxidative stress in

2 C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
various organs including the heart; this manifests as hypotrophy,apoptosis, and fibrosis [16]. The potential roles of inflammation and ox-idative stress in cardiovascular disorders suggest that molecules withanti-inflammatory and antioxidant properties may enhance the efficacyof treatment protocols designed to mitigate FFA-induced injury.
Curcumin (Cur) is a naturally occurring phytochemical compoundfound in turmeric (Curcuma longa), with a long history of use in tradi-tional Indiandiets andherbalmedicine [17]. It has several pharmacolog-ical and biological properties such as being an antioxidant, anti-inflammatory, anticancer, antimicrobial, antiviral and antifungal agent[17]. Many studies revealed that curcumin exerts a powerful free radicalscavenging effect with potent anti-inflammatory effects [18]. Its anti-inflammatory effect is mediated through multiple mechanisms involv-ing inhibition of various transcription factors such as NF-κB anddown-regulation of pro-inflammatory cytokines such as TNF-α and IL-6 [17]. In addition, curcumin was previously shown to up-regulate theexpression and activity of nuclear factor erythroid 2 (Nrf2) [19,20],whose downstream proteins were shown to have important functionsin protection against oxidative stress [21]. Curcumin also has protectiveeffects against several cardiovascular diseases [22].
With renewed interest in the pharmaceutical potential using naturalproducts with minimal side effects, many studies have been carried outin the exploration of using curcumin for biomedical purposes [17]. Theaim of our study is to examine whether curcumin is cardioprotectiveagainst FFA- and HFD-induced cardiac injury. Using cell culture studiesand a high fat in vivomodel, we have also explored curcuminmechanis-tically for its anti-inflammatory and antioxidant potential. Our resultsdemonstrated that curcumin treatment significantly ameliorated cardi-ac hypertrophy, apoptosis, andfibrosis associatedwith PA/HFD-inducedinjury. The cardiac protection from PA and HFD by curcumin was foundto be mediated most likely by activation of nuclear factor erythroid2-related factor 2 (Nrf2) and inhibiting NF-κB-actions.
2. Results
2.1. Curcumin prevents PA-induced ROS production and oxidative stress inH9C2 cells
Elevated circulating FFA levels increase oxidative stress in cardiovas-cular complications [23]. This promptedus to investigate the scavengingactivity of curcumin on PA-induced reactive oxygen species (ROS)production in H9C2 cardiac cells. According to the previous publications[24,25], we used PA at the concentration of 500 μM to stimulate H9C2cells. As evident from Fig. 1A and B, ROS generation was significantlyincreased with PA treatment at 500 μM, evidenced by increased ROS-positive dichlorodihydro fluorescent (DCF) intensity than compared tocontrol cells (PA untreated; DMSO only). Treatment with curcuminusing a 20 μM concentration significantly decreased ROS productionwith a confirmation using flow cytometry. The mean fluorescent inten-sity (MFI) values showed that curcumin significantly reduced PA-induced increases in ROS-positive cells (Fig. 1C).
Previous studies suggest that curcuminmaymediate its anti-oxidantactivity in part through the modulation of Nrf2. Hence, we investigatedprotein and mRNA levels of Nrf2 via western blot and RT-PCR respec-tively (Fig. 1D and E). Our results show significantly elevated levels ofNrf2 protein and mRNA in cells exposed to curcumin. We then assayedfor Nrf2-dependent antioxidant defense genes heme oxygenase-1 (HO-1), glutamate-cysteine ligase (GCLC), and NAD(P)H dehydrogenase,quinone (NQO-1). We found that mRNA expression of HO-1, GCLC,and NQO-1 followed the same pattern as Nrf2 with significantly in-creased levels following curcumin treatment (Fig. 1F–H). Similar resultswere observed in the protein level as evidenced in Fig. 1I–K. Theseresults indicate that the anti-oxidative effects of curcumin may resultfrom its ability to induce Nrf-2 and upregulate expression of down-stream anti-oxidant genes.
2.2. Anti-inflammatory effect of curcumin in H9C2 cells exposed to PA
Our next objective was to determine whether curcumin exhibitsanti-inflammatory activity in PA-treated H9C2 cells. Therefore, an im-munofluorescence assay was performed to detect the expression anddistribution of NF-κB p65 in H9C2 cells. Our results showed that NF-κB p65 accumulated in the nuclei of PA-treated cells (Fig. 2A). Thiswas supported by an electrophoretic mobility shift assay (EMSA) analy-sis (Fig. 2B) which showed increased DNA-binding activity of NF-κB innuclear fraction of PA-treated cells. Building on these results, we inves-tigated mRNA expression levels of NF-κB-activated inflammatory cyto-kines such as TNF-α, IL-1β and IL-6 (Fig. 2C–E). We found that theexpression of TNF-α, IL-1β and IL-6 was significantly increased in thePA-treated cells compared to control cells. Upon treatment withcurcumin, NF-κB activation was significantly attenuated as evidencedby reduced nuclear immunofluorescence and reduced DNA-bindingactivity of NF-κB in nuclear fraction. Curcumin also reduced the PA-induced mRNA expression of TNF-α, IL-1β and IL-6.
2.3. Effect of curcumin on PA-induced apoptosis in H9C2 cells
We further determined the protective effect of curcumin on PA-induced H9C2 cell apoptosis. As shown in Fig. 3A, PA induced apoptosisin H9C2 cells after only 24 h exposure. This was accompanied by mor-phological changes and decreased Hoechst staining of cells. We thenassayed for annexin V/PI to show that the relative percent of apoptoticcells and the number of annexin V positive cells were significantlyhigher in PA treated cells (Fig. 3B and C). Treatment with curcumin at20 μM profoundly attenuated apoptotic morphology and decreasedthe number of apoptotic cells as evidenced by Hoechst staining andflow cytometry (Fig. 3A–C). Furthermore, 16 h PA treatment increasedthe expression of several important pro-apoptotic proteins such ascleaved caspase-3 and Bax and decreased the expression of anti-apoptotic Bcl-2. Pretreatment with curcumin at 20 μM significantlyreversed these changes in apoptosis-related genes induced by PA(Fig. 3D).
2.4. PA-induced hypertrophy and fibrotic response are attenuated bycurcumin in H9C2 cells
The effect of curcumin on cardiac cell hypertrophywas examined bymeasuring the cell surface area using rhodamine phalloidin staining.Cells were pretreated with 20 μM curcumin for 1 h and then incubatedwith PA at 500 μM for 6 h. As shown in Fig. 4A and B, curcumin signifi-cantly suppressed PA-induced hypertrophy. Next, the mRNA level ofcardiac fibrosis marker gene transforming growth factor-β (TGF-β)was determined by RT-qPCR. Fig. 4C showed that the expression ofTGF-βwas significantly higher in PA-treated cells compared to the vehi-cle control and pretreatment with curcumin significantly inhibited theincreased expression of TGF-β.
2.5. Administration with curcumin did not affect the lipid profiles of HFD-fed mice
In vivo, we employed the HFD-fed mouse models to investigatewhether curcumin exhibits cardioprotective effects in obesity. Here,curcumin was used at the oral dosage of 50 mg/kg/day, according tothe previous studies on curcumin in HFD-fed obese mice [26]. Micewere fed with HFD for 8 weeks and then gavaged with curcumin(50 mg/kg/day) or vehicle for 8 weeks. At the end of the experimentalperiod, we measured the body weight and lipidemia indices. Asshown in Fig. 5A–D, body weight, and levels of serum TG, serum TCH,and serum LDL were significantly increased in the HFD-fed mice whencompared to control mice. However, oral administration with curcumindid not show any significant changes in the serum lipid profile and levelof HFD-fed mice (Fig. 5A–D).

Fig. 1. Curcumin (Cur) inhibits PA-induced ROS generation in H9C2 cells. (A–C) Representative staining images and flow cytometry assay for H2O2 levels. H9C2 cells that were pretreatedwith curcumin (20 μM) for 1 h were incubated with PA (500 μM) for 14 h, and then the DCFH-DA probe (2 μM) were loaded. ROS positive cells were detected using a fluorescence mi-croscope (A) with the quantitative data in means ± SE from 3 independent experiments (B), and using flow cytometry with mean fluorescence intensity (MFI) value of each group(C). (D) Western blot analysis for Nrf2 expression. H9C2 cells were pretreated with curcumin (20 μM) for 1 h and then incubated with PA (500 μM) for 14 h. Cells were lysated andthe protein extracts were processed for western blot analysis with GAPDH as a loading control. The column figures show the normalized optical density from the data in 4 independentexperiments. (E–H) Real-time qPCR analysis for Nrf2 gene and downstream gene expression. H9C2 cells pretreated with curcumin (20 μM) for 1 h were incubated with PA (500 μM) for6 h. Cells were harvested and total RNAs were extracted. Anti-oxidative genes Nrf2 (E), HO-1 (F), GCLC (G), and NQO-1 (H) were detected for real-time qPCR analysis using GAPDH as acontrol gene. (n = 5, * P b 0.05, ** P b 0.01, *** P b 0.001).
3C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
2.6. Effect of curcumin on oxidative stress and inflammation in the heart ofHFD mice
The inflammatory and oxidative indexes were determined in HFD-fed mice with or without curcumin administration. In the cell culturestudies, curcumin prevented PA-induced NF-κB activation. For NF-κBactivation, a key step is the degradation of IκB which allows transloca-tion of NF-κB p65 from the cytosol to the nucleus. Therefore, we firstassayed for IκB levels to determine whether curcumin alters NF-κB
activation in mice. As shown in Fig. 6A, protein levels of IκB-αwere sig-nificantly decreased in the heart of HFD group mice, indicating an acti-vation of NF-κB. Treatment of mice with curcumin restored the levelsof IκB-α in the heart tissues. Associated with the NF-κB alteration, weshowed that TNF-αwas increased in HFD-fedmice compared to controlmice and curcumin normalized the level of TNF-α (Fig. 6C). Similarresults were observed at the mRNA level. HFD increased the mRNAexpression of TNF-α, IL-1β, and IL-6 (Fig. 6C–E) and treatment withcurcumin significantly inhibited HFD-induced inflammatory cytokine

Fig. 2. Curcumin inhibits PA-induced inflammatory response in H9C2 cells. (A–B) Immunofluorescence assay for NF-κB p65 nuclear translocation and EMSA for NF-κB activity. H9C2 cellspretreatedwith curcumin (20 μM) for 1 hwere incubatedwith PA (500 μM) for 1 h. Cells were harvested and processed for p65 immunofluorescence staining (A) and the nuclear proteinswere extracted for EMSA (B) as described in theMaterials andmethods section. Representative images from 3 independent experiments were shown. (C–E) mRNA expression of pro-in-flammatory cytokines. H9C2 cells pretreated with curcumin (20 μM) for 1 h were incubated with PA (500 μM) for 6 h, then the cells were collected and the extracted total RNAs wereprocessed for real-time qPCR assay for TNF-α (C), IL-6 (D), and IL-1β (E) genes using GAPDH as a control gene. (n = 4, * P b 0.05, ** P b 0.01).
4 C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
expression. We then tested the effects of curcumin in HFD-inducedoxidative stress in the mouse heart. The data in Fig. 6F and G revealedthat Nrf2 expression was significantly increased in the hearts of HFD-fed mice. Treatment with 50 mg/kg curcumin also induced a significantincrease in the mRNA and protein expression of Nrf-2-downstreamanti-oxidant genes HO-1 and NQO-1 in the myocardium of HFD-fedmice (Fig. 6H–J).
2.7. Effect of curcumin on morphological changes and fibrosis in heart ofHFD-fed mice
To support the protective effect of curcumin in cardiac remodeling,hematoxylin and eosin (H&E) staining showed that HFD-fed mousehearts displayed structural abnormalities, including broken fibers andderanged cellular structures, while there was no significant evidenceof these abnormalities in the hearts ofHFD-fedmice that hadbeen treatedwith curcumin (Figs. 7A and 6B). Cardiac hypertrophy was characterizedwith increased cell surface area and expression of cardiac hypertrophicmarker genes such asα-myosin heavy chain (α-MyHC) and B-type natri-uretic peptide (BNP). In H&E stained sections, HFDmice showed a signif-icantly increased cardiomyocyte transverse cross-sectional area (Fig. 7A),consistent with the increased cell size (Fig. 7B) and the heart weight/tibialength (HW/TL) ratio (Fig. 7C). In addition, the mRNA expression of α-MyHC and BNP was also significantly increased in the hearts of HFD-fedmice (Fig. 7D–E). As shown in Fig. 7A–E, curcumin administration signif-icantly reversed the increased cardiomyocyte volume and HW/TL ratioand also inhibited the mRNA expression of BNP and α-MyHC. Thesefindings suggested that curcumin prevents the development of cardiachypertrophy in HFD-fed mice.
We further demonstrated the anti-fibrotic property of curcuminin vivo. Sirius red staining revealed a marked collagen accumulation inthe hearts of HFDmice, while curcumin treatment significantly reduced
the degree of collagen deposition (Fig. 7F). RT-PCR analysis revealedsignificant increases in the expression of pro-fibrotic gene connectivetissue growth factor (CTGF; Fig. 7G) and TGF-β (Fig. 7H) in HFD group.These changes were significantly blocked by the administration ofcurcumin. Next, mRNA levels of myocardial extracellular protein suchas collagen 1 and matrix metalloproteinase-9 (MMP-9) were signifi-cantly increased in the hearts of HFD mice (Fig. 7I–J). These changeswere also remarkably reversed by curcumin administration. Thechanged profile of cardiac collagen 1 gene (Fig. 7J) was consistentwith the evidence from the microscopic examination of the cardiactissue sections (Fig. 7F).
2.8. Effect of curcumin on the heart of HFD induced apoptosis
Finally, we examined the anti-apoptotic effects of curcumin in hearttissues of HFD mice. We observed a marked increase in pro-apoptoticgene Bax (Fig. 8A and B). Apoptosis was further confirmed by anincrease in TUNEL-positive cells (Fig. 8C and D). In parallel to ourin vitro studies,we show that treatment of HFDmicewith curcumin sig-nificantly prevented the activation of Bax and induction of the TUNELpositive cardiomyocytes. These findings suggested that curcumin atten-uates HFD-induced cardiomyocyte apoptosis, which may be partiallyrelated to its suppression of oxidative stress and inflammation.
3. Discussion
The relationship between obesity and cardiovascular disease hasbeen unequivocally established clinically and in experimentalmodels. High-fat diet intake and obesity trigger hyperlipidemia,hyperleptinemia, hyperinsulinemia, insulin resistance, elevated cir-culating cytokine, hypertension, and type 2 diabetes, and are alsoconsidered major risk factors for myocardial remodeling and injury

Fig. 3. Curcumin inhibits PA-induced cell apoptosis in H9C2 cells. (A) Representative images for cell and nucleus morphology. H9C2 cells pretreated with curcumin (20 μM) for 1 h wereincubatedwith PA (500 μM) for 24 h, then the cells were processed formorphology analysis using phage contrastmicroscope orwere processed for Hoechest staining assay as described intheMaterials andmethods section. (B–C) Flow cytometry assay for cell apoptosis. H9C2 cells pretreatedwith curcumin (20 μM) for 1 hwere incubatedwith PA (500 μM) for 16 h, then thecellswere processed for cell apoptosis analysis using FITC-annexinV and PI double staining. Representative flow cytometry imageswere shown (B)with thequantitative columnfigure forFITC-annexin V positive cells in means ± SE from 3 independent experiments (C). (D) Western bolt analysis for cleaved-casepase 3, Bax, and Bcl2. H9C2 cells pretreated with curcumin(20 μM) for 1 hwere incubatedwith PA (500 μM) for 16 h. The extracted total proteinswere processed for western blot analysis with GAPDH as a loading control. Blots are representativeand the column figures show the normalized optical density from the data more than 4 independent experiments. (n = 5, ** P b 0.01).
5C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
[27]. Recent studies have emphasized that oxidative stress andchronic inflammatory processes are important in the pathophysiologyof obesity-related cardiovascular disorder [4]. Uncontrolled productionof ROS and inflammatory cytokines induced by hyperlipidemia impairscellular functions and causes cell apoptosis in a variety of tissues includ-ing the heart [28]. Therefore, elucidating the mechanism by which hy-perlipidemia causes cardiac injury and discovering novel therapeuticagents are timely.
Natural products may help in the identification of bioactive leadcompounds and their development into drugs for treating oxidativeand inflammatory diseases. A number of phytochemicals, especiallypolyphenolic compounds, have been shown to possess anti-oxidantand anti-inflammatory properties and some have proved to be involvedin the attenuation of cardiac injury and cardiovascular protection [29].Curcumin is an important polyphenolic compound from C. longa. Accu-mulating evidence has demonstrated that the desirable preventive ortherapeutic effects of curcumin are associated with its antioxidant andanti-inflammatory properties [30]. Recently, Pongchaidecha A and col-leagues showed protective effects of curcumin in cardiac injury induced
by HFD; however, they failed to show the molecular mechanism for itsmode of action [31]. Since the mechanism of curcumin effect is notfully known,we devised this study to offer insight in its regulatory func-tions. We found that curcumin treatment significantly down-regulatedpathological indices of HFD-induced cardiac injury. However, curcuminadministration at 50 mg/kg/day did not decrease the HFD-inducedhyperlipidemia profile (Fig. 5), indicating that the primary target ofcurcumin action is on cardiac protection in HFD-fed mice, rather thanaltering lipid levels.
Hyperlipidemia is defined as a condition of high levels of cholesterol,triglycerides, LDL, and free fatty acids, which can increase the risk ofheart disease, stroke, and other health problems. Lipotoxicity is a termused variably to describe cellular injury and death caused by free fattyacids, their metabolites, and even triglyceride, although triglyceride isgenerally found to be relatively inert. Recent evidences have indicatedhepatic lipotoxicity mostly attributed to free fatty acids but not triglyc-erides [32]. Plasma FFA levels are usually elevated in obesity and chron-ically elevated plasma FFA has many pathophysiological consequences.Among the elements of hyperlipidemia, FFAs plays important roles in

Fig. 4. Curcumin inhibits PA-induced hypertrophy and fibrosis in H9C2 cells. H9C2 cells pretreated with curcumin (20 μM) for 1 h were incubated with PA (500 μM) for 6 h. (A–B) Cellswere processed for cell surface area analysis using rhodamine–phalloidin staining as described in theMaterials andmethods section. Representative staining imageswere shown (A)withthe quantitative data for cell size of more than 100 randomly selected cells in 3 independent experiments (B). (C) Cells were harvested and total RNAswere extracted. ThemRNA expres-sion of TGF-β was detected by real-time qPCR with GAPDH as a control gene (n = 3). (* P b 0.05, ** P b 0.01, *** P b 0.001).
6 C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
the development of obesity-related complications, and FFAs haveemerged as a major inducer in obesity-related atherosclerotic vasculardiseases [33].
Palmitate is a major saturated FFA in plasma for the stimulation ofROS production and inflammatory cytokine expression in cultured aor-tic smooth muscle cells and endothelial cells [34–36]. Several reportsalso showed that plasma FFAs may affect vascular functions [37]. Here,we observed a significant augmentation of ROS and oxidative stress,when H9C2 cells were treated with PA (Fig. 1A–C). Many genes in-volved in oxidative stress have been confirmed to be directly or indi-rectly controlled by Nrf2 [21]. When activated, Nrf2 is bound toantioxidant transcription elements in the promoter regions of phase 2
Fig. 5. The effects of curcumin treatment on the profiles of bodyweight and serum lipids inHFD-serum LDL (D) was not affected by curcumin administration. TG = triglyceride, TCH = total ccontrol group).
detoxification enzyme genes and certain antioxidant genes. Nrf2 in-creases their expression, and leads to cellular resistance to oxidativestress [21]. Tanaka et al. have reported that Nrf2 inhibits the oxidativestress in the liver induced by FFA accumulation inHFD-fedmice [38]. In-terestingly, curcumin has an anti-oxidant effect and in vitro studieshave indicated its action on triggering Nrf2 signaling [39]. It has beenshown that curcumin activates the Nrf2 pathway to protect againstROS-mediated damage in several models [40–42]. Recently, it wasfound that short-term treatment of curcumin in HFD-fedmice effective-ly amelioratesmuscular oxidative stress by activatingNrf2 function [26]. However, it is not known whether curcumin protects the heart fromHFD-induced injury via Nrf2 activation. Our results showed that
fedmice. TheHFD-induced increase in bodyweight (A), serumTG (B), serumTCH (C), andholesterol, LDL = low density lipoprotein (n = 7 in each group, * P b 0.05, ** P b 0.01, vs.

Fig. 6. Curcumin attenuates HFD-induced inflammatory and oxidative pathways inmouse hearts. After the HFD-fed mice were killed, the heart tissues in control group (Ctrl), HFD group,and HFD+ curcumin group were collected. (A) Tissues were homogenated to detect IκBα level by western blot method. GAPDHwas used as a loading control. The column figures showthe normalized optical density of respective proteins (n = 6) (B and F). Heart tissues (5 μm section) from each group (n = 4) were processed for anti-TNF-α (B) and anti-Nrf2(F) immunofluorescence staining as described in the Materials and methods section. Representative staining images were shown (C–E and G–I). Curcumin administration reduced indi-cated gene expression inHFD-induced hearts. Heart tissues from each groupwere individually processed for RNA extraction and RT-qPCR analysis. ThemRNA levels of TNF-α (C), IL-6 (D),IL-1β (E), Nrf2 (G), HO-1 (H), and NQO-1 (I) were normalized by β-actin (n = 5–8) (* P b 0.05, ** P b 0.01).
7C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
treatment with curcumin increased the expression of Nrf2 both in vitroand in vivo (Figs. 1D, E and 6G).We further found that the expression ofNrf2-downstream genes GCLC, HO-1, and NQO-1 are also significantlyincreased by curcumin treatment (Figs. 1F–H, 6H and I). Therefore,these results suggest that curcumin-induced Nrf2 activation is a possi-blemechanismagainst HFD-induced oxidative stress to attenuate cardi-ac injury.
Several reports have shown that obesity is associated with a state ofchronic, low-grade inflammation, suggesting that inflammationmay bea potential mechanism whereby obesity leads to cardiovascular disor-der [2]. Elevated level of FFAs is also strongly associated with the pro-gression of inflammation through production of pro-inflammatorycytokines [43]. In macrophages, FFAs trigger inflammatory responsesvia toll-like receptor 4 and peroxisome proliferator-activated receptor
activation which promotes inflammatory gene expression by triggeringNF-κB activation [43,44]. Among the intracellular signaling system in-volved in the regulation of inflammatory responses, the transcriptionalfactor NF-κB has a special position. The upstream IKKα interacts withIκB-α and specifically phosphorylates IκBα to promote its degradation.The dissociation of IκB from the inactive cytoplasmic complex leads tothe translocation of the active subunit NF-κB p65 from the cytosolic tonuclear fractions, which binds to certain DNA sites and triggers inflam-matory gene expression. Curcumin administration has been associatedwith a positive outcome in many chronic inflammatory diseases [45].It exerts its anti-inflammatory effects via several mechanisms. Forexample, curcumin inactivatesNF-κB, resulting in thedecreased expres-sion of TNF-α, IL-1 and IL-6 [45]. Our studies showed that PA increasesp65 translocation and NF-κB activity in cultured cardiac cells, while

Fig. 7. Curcumin administration improved cell histological abnormalities, hypertrophy, and fibrosis in HFD-induced hearts. (A and F) Heart tissues were sectioned at 5 μm and the slideswere processed for staining assay (n=5 in each group). Representative images from longitudinal and transverse H&E staining (A) and Sirius red staining (F) for heart tissueswere shown.All images were obtained bymicroscopewith 400× amplification. (B) Quantitative data of the cell size of more than 20 cells in each group frommyocardial transverse H&E stainingwereshown. (C) Data for the ratio of heart weight (HW) to tibia length (TL) (D–E and G–J). Curcumin administration reduced indicated gene expression in HFD-induced hearts. Heart tissuesfrom each groupwere individually processed for RNA extraction and RT-qPCR analysis. ThemRNA levels ofα-MyHC (D), BNP (E), CTGF (G), TGF-β (H),MMP-9 (I), and collagen 1 (J) werenormalized by β-actin (n = 5–8) (* P b 0.05, ** P b 0.01).
8 C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
HFD decreases IκB-α in the heart tissues of mice. Curcumin significantlyinhibited NF-κB activation induced by PA/HFD thus alleviating the ex-pression of inflammatory cytokines like TNF-α, IL-6, and IL-1β, bothin vitro and in vivo. This indicates that curcumin inhibits PA-inducedcardiac inflammation via inactivation of NF-κB.
It is well known that oxidative stress and inflammation in cells arestrongly associated with the induction of apoptosis [46,47]. An in vitrostudy revealed that curcumin protects smooth muscle cells against
apoptosis evoked by oxidative stress [48]. As a result of oxidative insultand inflammation, we showed that cardiac cells undergo apoptosiswhich was evidenced by the enhanced number of apoptotic cells, anincreased expression of Bax, and the decreased expression of anti-apoptotic protein Bcl-2 both in vitro and in vivo (Figs. 3 and 8A–D). Inrespect to the above, we investigated the protective effects of curcuminon cell morphology and the apoptotic markers in PA-treated cells. Ourfindings support that curcumin treatment decreases cardiomyocyte

Fig. 8. Curcumin administration improved cell apoptosis in HFD-induced hearts. (A) Heart tissues were sectioned at 5 μmand the slides were processed for anti-Bax immunofluorescencestaining (n= 5 in each group). (B) A quantitative analysis of relative amount of Bax in A. (C) Heart tissueswere sectioned at 5 μmand the slideswere processed for TUNEL assay to detectapoptotic cells (n = 5 in each group). (D) The column figures show the relative TUNEL positive cell number (* P b 0.05, *** P b 0.001). (E) A schematic illustration for the prevention ofcurcumin from FFA/HFD-induced injury in cardiomyocytes and hearts.
9C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
apoptosis. It is reasonable to speculate curcumin alleviated apoptosis byalleviating the oxidative stress and inhibiting the NF-κB activation.
Oxidative stress and inflammation are also implicated in the devel-opment and progression of cardiac hypertrophy [49,50]. Increased cellsurface and the increased expression of proteins such as α-MyHC andBNP are regarded as important phenotypic and molecular markers forcardiac hypertrophy, respectively. Recently, curcumin has been shownto attenuate the lipopolysaccharide-induced cardiac hypertrophy in arodent model [51]. Our data showed that PA/HFD caused a significantincrease in the cell/heart volume and increasedα-MyHCand BNP levels,while curcuminwas capable of attenuating the hypertrophic phenotypeinduced by PA/HFD (Figs. 4A–B and 7A–E).
Cardiac fibrosis is a classical feature of pathological hypertrophy andis characterized by the expansion of the extracellular matrix due to theaccumulation of collagen. The relationship between inflammation andcardiac fibrosis has been well established [52]. Moreover, increased ox-idative stress represents a putative feature in obesity and was furthershown to participate in the progression of cardiacfibrosis either directlyor indirectly via the activation of TGF-β [53]. In this regard, attenuationof the fibrotic response would be expected in the heart of curcumin-treated mice fed HFD based on its established anti-inflammatory andantioxidant properties [22]. In the present study, an extensive fibroticresponse was observed in the heart of HFD mice which was evidencedby the increased mRNA expression of TGF-β, CTGF, collagen 1 andMMP-9 (Fig. 7G–J). Collectively, these data were consistent with previ-ous studies demonstrating the presence of a reactivefibrotic response inthemyocardium of various experimental animalmodels of obesity [54].Our studies showed that curcumin inhibited the increased expression of
both TGF-β and CTGF in HFD mice thereby inhibiting the expression ofmyocardial extracellular matrix proteins such as collagen 1. These find-ings prove the anti-fibrotic activity of curcumin in the hearts of HFDmice.
In conclusion, the findings of the present study, both in vitro andin vivo, reinforce the defensive role of curcumin against oxidative stress,inflammation, apoptosis, hypertrophy and fibrosis. The beneficial ac-tions of curcumin are closely associated with its ability in increasingNrf2 and inhibiting NF-κB (Fig. 8E). This study clearly suggests the ther-apeutic application of curcumin in the treatment of obesity-related car-diac disorders. In addition,Nrf2 andNF-κB, in the regulation of oxidativestress and inflammation respectively, mediate the hypertrophic,apoptotic, and fibrotic effects of both PA-stimulated cardiac cells andHFD-induced hearts (Fig. 8E). These results provide a deeper under-standing in the regulatory role of Nrf2 and NF-κB for hyperlipidemia-induced cardiac injury, indicating that theymay be important therapeu-tic targets for obesity-related disorders.
4. Materials and methods
4.1. Chemicals and reagents
Palmitate (PA) and curcumin were purchased from Sigma-Aldrich(St. Louis, MO, USA). Stock solutions of 5 mM PA/10% BSA were pre-pared and stored at−20 °C. Stock solutions were heated for 15 min at55 °C and then cooled to room temperature prior to use. Curcuminwas dissolved in DMSO for in vitro experiments and in CMCNa (1%)for in vivo experiments. Antibodies used in the experiments were

10 C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
purchased from the following suppliers: Nrf2, Bcl-2-like protein 4 (Bax),B-cell lymphoma 2 (Bcl2), NF-κB p65, inhibitor of κB (IκB), and B-typenatriuretic peptide (BNP) from Santa Cruz Biotechnology (Santa Cruz,CA), TNF-α from Abcam (Cambride, MA), anti-cleavaged caspase-3from Cell Signaling Technology (Danvers, MA) and horseradishperoxidase-conjugated anti-rabbit secondary antibodies from SantaCruz. Enhanced chemiluminescence reagent, FITC annexin V apoptosisdetection kit, and one-step TUNEL apoptosis assay kit were obtainedfrom Beyotime (Beijing, China).
4.2. Cell culture assays
H9C2 embryonic rat heart-derived cell line was obtained from theShanghai Institute of Biochemistry and Cell Biology (Shanghai, China)and cultured in DMEM/F12 medium (Gibco, Eggenstein, Germany)containing 2.25 g/L glucose supplemented with 10% FBS, 100 U/mL ofpenicillin, and 100 mg/mL of streptomycin in a humidified atmosphereof 5% CO2 at 37 °C. Following treatments, cells were subjected to variousassays.
The level of intracellular reactive oxygen species (ROS) was deter-mined using 2,7-dichlorodihydro fluorescent diacetate (DCFH-DA).Cells were pretreated with curcumin (20 μM) for 1 h and followed byincubation with PA (500 μM) for 14 h. Then, cells were loaded with2 μmol/L DCFH-DA, incubated at 37 °C for 30 min, and washed 3times. Fluorescence intensity was measured with a fluorescencemicro-scope, with excitation wavelengths of 488 nm. We then collected cellsfor flow cytometry using FACS caliber (BD Science, CA) and Cell Questsoftware.
4.3. Immunofluorescence staining
Immunofluorescence was used for cell surface measurements, apo-ptosis determination, and NF-κB activation. To determine cell apoptosis,we harvested cells following treatments and stainedwith annexin V andpropidium iodide (PI). Flow-cytometric analysis was then performedusing FACS caliber (BD, CA) using Cell Quest software. For morphologyand cell surface measurements, we fixed cells with 4% paraformalde-hyde, permeabilized with 0.1% Triton X-100, and stained with rhoda-mine phalloidin at a concentration of 50 μg/mL for 30 min at roomtemperature for fluorescence microscopy. Treated cells were alsostained with Hoechst 33342 dye (Beyotime Biotech, Nantong, China)for morphological assessment.
Immunofluorescent detection for NF-κB was carried out by fixingcells in 100% methanol at −20 °C for 5 min. After fixation and perme-abilization, cells were washed twice with PBS containing 1% bovineserum albumin (BSA), and incubated with anti-p65 antibody (1:200)overnight at 4 °C. FITC-conjugated secondary antibody (1:200) wasused for detection. The stained sections were then viewed under theNikon fluorescence microscope (400× amplification; Nikon, Japan).
4.4. Animal experiments
Male C57BL/6mice (8 weeks of age)weighing 18–22 gwere used inthis study. The animals were obtained from the Animal Center ofWenzhou Medical University. All animal studies decried in this workhave been approved by theWenzhou Medical University Animal Policyand Welfare Committee (approved documents: wydw2014-0058).Twenty-one mice were housed in an environmentally controlled roomat 22 ± 2.0 °C and 50% ±5% humidity with a 12-h:12-h light/darkcycle and fed food and water ad libitum. Mice were randomly dividedinto three weight-matched groups. Seven mice were fed standard ani-mal chow and served as a normal control group (ND). The remaining14micewere fed with HFD (fromMediscience Diets Co. Ltd., Yangzhou,China) for 8 weeks. After 8 weeks of feeding, HFD-fed mice werefurther divided into two groups, HFD group (n = 7) and HFD pluscurcumin treated group (n = 7). Curcumin was given daily by oral
gavage at a dose of 50 mg/kg in 1% CMCNa solution for 8 weeks. Micein the ND and HFD groups were gavaged with vehicle only. At the endof experimental period, all the animals were euthanized with CO2. Thebody weight was recorded and blood samples were collected and cen-trifuged at 4 °C for 10 min for collecting serum. The heart was excisedaseptically, blotted dry and theweightwas recorded followed by imme-diate freezing in liquid nitrogen and then stored at −80 °C beforefurther analyses.
4.5. Western blot analysis and electrophoretic mobility shift assays (EMSA)
Cells or tissue homogenates were lysed and protein concentrationswere determined by using the Bradford protein assay kit (Bio-Rad,Hercules, CA). Lysates were then analyzed through western blotting,and the immunoreactive bands were visualized by using ECL kit (Bio-Rad, Hercules, CA).
For EMSA, nuclear extracts were prepared using the nuclear andcytoplasmic protein extraction kit (KeyGen Biotech) and proteinconcentrations were determined. A total of 10 μg nuclear extractswere incubated with NF-κB oligonucleotide in a buffer containing0.25 mg/mL of poly(dI.dC), 50 mmol/L Tris–HCl (pH 7.5), 250 mmol/LNaCl, 5 mmol/L MgCl2, 2.5 mmol/L DTT, 2.5 mmol/L EDTA and 20%glycerol (total volume of 30 mL) for 30 min at room temperature. Thereaction was stopped by the addition of 1/10 of the reaction volume of10× gel loading buffer (250 mmol/L Tris–HCl, 0.2% bromophenol blue,0.2% xylene cyanol, 40% glycerol). The reactions were run on a non-denaturing (4% acrylamide 29:1 acrylamide to bisacrylamide) gel, atroom temperature with 100 V until the dye was about 3 cm from thebottom of the gel. The running buffer was 0.5× TBE (45 mmol/L Tris–HCl, 45 mmol/L boric acid, 1 mmol/L EDTA). After electrophoresis,DNA–protein complex was transferred to a nylon membrane, andcross-linked. Then the biotinylated-labeled DNA was detected bychemiluminescence. NF-κB consensus oligonucleotides (double strand-ed DNA) were: 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; 3′-TCA ACTCCC CTG AAA GGG TCC G-5′.
4.6. Real-time quantitative PCR
Total RNA was isolated from cells or tissues (50–100 mg) usingTRIzol (Life Technologies, Carlsbad, CA). Reverse transcription andquantitative PCR (RT-qPCR) was performed using MMLV Platinum RT-qPCR kit (Life Technologies). Real-time qPCR was carried out using theEppendorf Real plex 4 instrument (Eppendorf, Hamburg, Germany).Primers for genes including TNF-α, IL-6, IL-1β, transforming growthfactor-β (TGF-β), Nrf2, heme oxygenase-1 (HO-1), NAD(P)H dehydro-genase, quinone (NQO-1), glutamate-cysteine ligase (GCLC), collagen1, connective tissue growth factor (CTGF), matrix metalloproteinase-9(MMP-9), a-a-myosin heavy chain (a-MyHC), BNP, and β-actin wereobtained from Life Technologies. The primer sequences used areshown in Table S1. The relative amount of each gene was normalizedto the amount of β-actin.
4.7. Histological analyses
Excised heart tissue specimenswere fixed in 10% formalin processedin graded alcohol, xylene, and then embedded in paraffin. Paraffinblocks were sliced into sections of 5 μm in thickness. After rehydration,the sections were stained with hematoxylin and eosin (H&E). To evalu-ate the histopathological damage, each image of the sections was cap-tured using a light microscope (200× or 400× amplification, Nikon,Japan). Sectionswere also stained with 0.1% Sirius red F3B and 1.3% sat-urated aqueous solution of picric acid to evaluate the type IV collagendeposition.
To detect apoptosis, tissue sections were used for the terminaldeoxynucleotidyl transferase-mediated FITC-dUTP nick end labeling(TUNEL; one step TUNEL apoptosis assay kit, Beyotime, Beijing, China).

11C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
TUNEL positive cells were imaged under a fluorescence microscope(400× amplification; Nikon, Japan).
4.8. Immunofluorescence staining of tissues
Paraffin sections (5 μm) were deparaffinized and rehydrated, andthen subjected to antigen retrieval in 0.01 mol/L citrate buffer (pH 6.0)by microwaving. After blockingwith 5% BSA, the sections were incubat-ed with anti-Bax antibody (1:500), anti-TNF-α antibody (1:500), oranti-Nrf2 antibody (1:500) overnight at 4 °C, followed by the secondaryantibody (1:300; Santa Cruz). The nucleus was stained with DAPI andsections were then viewed under the Nikon fluorescence microscope(400× amplification; Nikon, Japan).
4.9. Measurements of the level of serum lipid
The components of serum lipid including the total triglyceride (TG),total cholesterol (TCH), and low density lipoprotein (LDL), were detect-ed using commercial kits (Nanjing Jiancheng Bioengineering Institute,Jiangsu, China).
4.10. Statistical analysis
The results were expressed asmean± SEM and statistically evaluat-ed using one-way ANOVA followed by multiple comparisons test withBonferroni correction using GraphPad Prism version 5.0 for Windows(GraphPad Software, Inc., San Diego, CA). P b 0.05 was consideredstatistically significant.
Disclosures
None. All the authors declare no competing financial interest.
Acknowledgments
This study was supported by the Natural Science Funding of China[21272179 to G.L., 81200572 to J.W., and 81261120560 to Y.Z.], theCIHR China–Canada Joint Health Research Initiative (81261120560)[to Z.K. and S.C.], the High-level Innovative Talent Funding of ZhejiangDepartment of Health (604090352-029) [to G. L. and C.Z.], and theZhejiang Key Group in Scientific Innovation [2010R50042 to X.L.].
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yjmcc.2014.10.002.
References
[1] Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu RevImmunol 2011;29:415–45.
[2] Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk.Clin Pharmacol Ther 2010;87:407–16.
[3] Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7.[4] Ndisang JF, Vannacci A, Rastogi S. Oxidative stress and inflammation in obesity,
diabetes, hypertension, and related cardiometabolic complications. Oxid Med CellLongev 2014;2014:506948.
[5] Boden G. 45Obesity, insulin resistance and free fatty acids. Current opinion in endo-crinology, diabetes, and, obesity. 2011;18:139.
[6] Manrique C, DeMarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, et al. Obesity andinsulin resistance induce early development of diastolic dysfunction in young femalemice fed a western diet. Endocrinology 2013;154:3632–42.
[7] Knowles CJ, Cebova M, Pinz IM. Palmitate diet-induced loss of cardiac caveolin-3: anovel mechanism for lipid-induced contractile dysfunction. PLoS One 2013;8:e61369.
[8] Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab2012;15:585–94.
[9] Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscleand liver. Curr Diab Rep 2006;6:177–81.
[10] Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, et al. Free fatty acidsproduce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes 2005;54:3458–65.
[11] Park S-Y, Kim J-E, Ahn M-W, Kim Y-W, Kim J-Y. AICAR inhibits palmitate-inducedapoptosis in osteoblast via increase in ERK. Diabetes 2007;56:A236–7.
[12] Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitatebut not oleate exposure induces endoplasmic reticulum stress, which may contrib-ute to INS-1 pancreatic β-cell apoptosis. Endocrinology 2006;147:3398–407.
[13] Yamagishi S, Okamoto T, Amano S, Inagaki Y, Koga K, Koga M, et al. Palmitate-induced apoptosis of microvascular endothelial cells and pericytes. Mol Med 2002;8:179–84.
[14] Gambert S, Vergely C, Filomenko R, Moreau D, Bettaieb A, Opie LH, et al. Adverseeffects of free fatty acid associated with increased oxidative stress in postischemicisolated rat hearts. Mol Cell Biochem 2006;283:147–52.
[15] Feillet-Coudray C, Sutra T, Fouret G, Ramos J, Wrutniak-Cabello C, Cabello G, et al.Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of poly-phenols: involvement of mitochondrial and NAD(P)H oxidase systems. Free RadicBiol Med 2009;46:624–32.
[16] van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2diabetic cardiomyopathy. Cardiovasc Res 2011;92:10–8.
[17] Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice:from bedside to bench and back. Biotechnol Adv 2014;32:1053–64.
[18] Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. CurrPharm Des 2013;19:2032–46.
[19] Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1expression and protects rat brains against focal ischemia. Brain Res 2009;1282:133–41.
[20] Wu J, Li Q, Wang X, Yu S, Li L, Wu X, et al. Neuroprotection by curcumin in ischemicbrain injury involves the Akt/Nrf2 pathway. PLoS One 2013;8:e59843.
[21] Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol2013;53:401–26.
[22] WongcharoenW, Phrommintikul A. The protective role of curcumin in cardiovascu-lar diseases. Int J Cardiol 2009;133:145–51.
[23] Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, et al. Nebivololimproves diastolic dysfunction and myocardial remodeling through reductions inoxidative stress in the Zucker obese rat. Hypertension 2010;55:880–8.
[24] Cetrullo S, Tantini B, Flamigni F, Pazzini C, Facchini A, Stefanelli C, et al. Antiapoptoticand antiautophagic effects of eicosapentaenoic acid in cardiac myoblasts exposed topalmitic acid. Nutr 2012;4:78–90.
[25] Egnatchik RA, Leamy AK, Jacobson DA, Shiota M, Young JD. ER calcium releasepromotes mitochondrial dysfunction and hepatic cell lipotoxicity in response topalmitate overload. Mol Metabol 2014;3:544–53.
[26] He HJ, Wang GY, Gao Y, LingWH, Yu ZW, Jin TR. Curcumin attenuates Nrf2 signalingdefect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice.World J Diabetes 2012;3:94–104.
[27] Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascu-lar disease. Nature 2006;444:875–80.
[28] Wende AR, Symons JD, Abel ED. Mechanisms of lipotoxicity in the cardiovascularsystem. Curr Hypertens Rep 2012;14:517–31.
[29] Vasanthi HR, ShriShriMal N, Das DK. Phytochemicals from plants to combat cardio-vascular disease. Curr Med Chem 2012;19:2242–51.
[30] Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curryspice on the path to cancer treatment. Molecules 2011;16:4567–98.
[31] Pongchaidecha A, Lailerd N, Boonprasert W, Chattipakorn N. Effects of curcuminoidsupplement on cardiac autonomic status in high-fat-induced obese rats. Nutr 2009;25:870–8.
[32] Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholicsteatohepatitis: the central role of nontriglyceride fatty acidmetabolites. Hepatology2010;52:774–88.
[33] Kankaanpaa M, Lehto HR, Parkka JP, KomuM, Viljanen A, Ferrannini E, et al. Myocar-dial triglyceride content and epicardial fat mass in human obesity: relationship toleft ventricular function and serum free fatty acid levels. J Clin Endocrinol Metabol2006;91:4689–95.
[34] Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, et al. Activationof NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol2009;29:1370–5.
[35] Quan J, Liu J, Gao X, Liu J, Yang H, Chen W, et al. Palmitate induces interleukin-8expression in human aortic vascular smooth muscle cells via Toll-like receptor 4/nuclear factor-kappaB pathway (TLR4/NF-kappaB-8). J Diabetes 2014;6:33–41.
[36] Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose leveland free fatty acid stimulate reactive oxygen species production through proteinkinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabe-tes 2000;49:1939–45.
[37] de Kreutzenberg SV, Crepaldi C, Marchetto S, Calo L, Tiengo A, Del Prato S, et al.Plasma free fatty acids and endothelium-dependent vasodilation: effect of chain-length and cyclooxygenase inhibition. J Clin Endocrinol Metab 2000;85:793–8.
[38] Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther 2008;325:655–64.
[39] Zhao SG, Li Q, Liu ZX,Wang JJ,Wang XX, QinM, et al. Curcumin attenuates insulin resis-tance in hepatocytes by inducing Nrf2 nuclear translocation. Hepatogastroenterology2011;58:2106–11.
[40] Carmona-Ramirez I, Santamaria A, Tobon-Velasco JC, Orozco-Ibarra M, Gonzalez-Herrera IG, Pedraza-Chaverri J, et al. Curcumin restores Nrf2 levels and preventsquinolinic acid-induced neurotoxicity. J Nutr Biochem 2013;24:14–24.

12 C. Zeng et al. / Journal of Molecular and Cellular Cardiology 79 (2015) 1–12
[41] Soetikno V, Suzuki K, Veeraveedu PT, Arumugam S, Lakshmanan AP, Sone H, et al.Molecular understanding of curcumin in diabetic nephropathy. Drug Discov Today2013;18:756–63.
[42] Soetikno V, Sari FR, Lakshmanan AP, Arumugam S, Harima M, Suzuki K, et al.Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnantkidney through the Nrf2–keap1 pathway. Mol Nutr Food Res 2013;57:1649–59.
[43] Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fattyacid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab2012;15:518–33.
[44] Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation.Trends Endocrinol Metab 2012;23:351–63.
[45] Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent ofCurcuma longa: a review of preclinical and clinical research. Altern Med Rev 2009;14:141–53.
[46] Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K,Wang X, et al. Mechanisms of celldeath in oxidative stress. Antioxid Redox Signal 2007;9:49–89.
[47] Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis inpancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol2005;77:587–97.
[48] Kang ES, Woo IS, Kim HJ, Eun SY, Paek KS, Kim HJ, et al. Up-regulation of aldosereductase expression mediated by phosphatidylinositol 3-kinase/Akt and Nrf2 is in-volved in the protective effect of curcumin against oxidative damage. Free Radic BiolMed 2007;43:535–45.
[49] SeddonM, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hyper-trophy and heart failure. Heart 2007;93:903–7.
[50] Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiachypertrophy. Hypertension 2008;51:1345–51.
[51] Chowdhury R, Nimmanapalli R, Graham T, Reddy G. Curcumin attenuation of lipo-polysaccharide induced cardiac hypertrophy in rodents. ISRN Inflammation, 2013;2013 539305.
[52] Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. KidneyInt 2010;78:S22–6.
[53] Koli K, Myllarniemi M, Keski-Oja J, Kinnula VL. Transforming growth factor-betaactivation in the lung: focus on fibrosis and reactive oxygen species. AntioxidRedox Signal 2008;10:333–42.
[54] Carroll JF, Tyagi SC. Extracellular matrix remodeling in the heart of thehomocysteinemic obese rabbit. Am J Hypertens 2005;18:692–8.

![The host immune response in respiratory virus infection ... · (IFN-α/β receptor), activating the JAK/STAT pathway [3]. ... croup, tonsilitis Seasonal influenza Sialic acids Fever,](https://static.fdocument.org/doc/165x107/5c7b1b0809d3f277748b45cf/the-host-immune-response-in-respiratory-virus-infection-ifn-receptor.jpg)











![CDR FoodLab Line [FOODLAB - Brownstone Asia-Tech 2 of 9 [OxiTester] (Code CDR - 225006Z01) Analysis system for test of Acidity (FFA), Peroxides (PV) and Poliphenols/stability index](https://static.fdocument.org/doc/165x107/5ea63c60ff517674815cb116/cdr-foodlab-line-foodlab-brownstone-asia-2-of-9-oxitester-code-cdr-225006z01.jpg)