Chemical Reactions
description
Transcript of Chemical Reactions

CHEMICAL
REACTIONS
LEVEL 1 –
CH
APTER 10
Reactant A
Reactant B
Product(s)

CHEMICAL REACTIONS:Reactants → products
Symbols:
(s)
(l)
(g)
(aq)
Δ
Catalyst
Descriptive sentence → Word Equation (Names → Formulas) → Chemical Equation

BALANCING CHEMICAL EQUATIONSExamples:
A. Ca + H3PO4 Ca3(PO4)2 + H2
B. KBrO3 KBr + O2
C. (NH4)2CO3 + NaOH Na2CO3 + NH3 + H2O

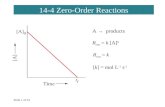
5 TYPES OF CHEMICAL REACTIONS:
1. Combination (Synthesis): only 1 product
2. Decomposition: only 1 reactant

3. Single-Replacement
Activity Series of Metals
Reactivity Trend in Periodic Table for Halogens

4. Double-Replacement

5. Combustion
Complete:
Incomplete:

WILL SOMETHING PRECIPITATE?Always soluble (aq): ionic compounds containing…
group 1 alkali metals, ammonium, nitrate, chlorate
Soluble EXCEPT:
chloride = soluble, unless containing Pb, Ag, Hg “the big three”
sulfate = soluble, unless containing Pb, Ag, Hg, Ca, Sr, Ba
NOT soluble (unless meets conditions above):
carbonate, phosphate, chromate, sulfide, hydroxide


TEST REVIEW: Matching / Multiple ChoiceWord / FormulaBalance / Type
Given: Periodic table, activity series of metals, solubility rules
Cu(s) + AgNO3(aq) → copper (II)…Type?Balance?
Lead (II) sulfate + nitric acid → Predict states?Type?Balance?