Chapter 4 Aromatic compounds - I am...
Transcript of Chapter 4 Aromatic compounds - I am...
-
1
Chapter 4Aromatic compounds
-
2
BenzeneBenzene: planar, ring, all atoms have p orbitals, 3 pairs of p electrons.
• Has six identical carbon–carbon bonds
• Each π electron is shared by all six carbons
• The π electrons are delocalized
-
3
Resonance Contributors and the Resonance Hybrid of Benzene
-
4
Nomenclature of Monosubstituted Benzenes
Some are named by attaching “benzene” after the name of the substituent
-
5
-
6
When a benzene ring is a substituent, it is called a phenyl group
A benzene ring with a methylene group is called a benzylgroup
-
7
With the exception of toluene, benzene rings with an alkylsubstituent are named as alkyl-substituted benzenes or as phenyl-substituted alkanes
Aryl group (Ar) is the general term for either a phenylgroup or a substituted phenyl group
-
8
In disubstituted benzenes, the relative positions of the two substituents are indicated by numbers or by prefixes
Nomenclature of Substituted Benzenes
-
9
The two substituents are listed in alphabetical order
-
10
If one of the substituents can be incorporated into a name, that name is used and the incorporated substituent is given the 1-position
-
11
Naming Polysubstituted Benzenes
The substituents are numbered in the direction that results in the lowest possible number
-
12
The incorporated substituent is given the 1-position; the ring is numbered in the direction that yields the lowestpossible number
-
13
The Resonance Energy of Benzene
-36 kcal/mole
+H2
+3H2
+3H2 -49.8 kcal/mole-28.6 kcal/mole
-85.8 kcal/mole
(28.6 × 2)
calculated
RESONANCE ENERGY
benzene
1,3,5-cyclohexatrienehypothetical
(-208 kJ)(-120 kJ)
(-360 kJ)
(- 152 kJ)
-
14
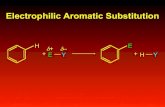
Electrophilic Aromatic Substitution Reactions
-
15
Electrophilic Aromatic Substitution
Aromatic ring systems react with electrophiles differently than alkenes.
H+ E+
E+ H+
+ E+Nu-E
Nu
We obtain a substitution rather than an addition product.
-
16
Mechanism
H+ E+
First, we get a carbocation intermediate.At the carbon that adds the electrophile, rehybridization (sp2 sp3) takes place. Molecule loses aromatic character.
H
EEH
-
17
Mechanism
Carbocation is resonance stabilized.
H
EEH
H
EEH
H
EEH
-
18
Mechanism
H
EEH
+
Nu- E
NuHH
E
+ HB
B-
The electron-rich part of the reagent can act either as nucleophile in an addition reaction or as a base to remove a proton.
-
19
-
20
Mechanism
The two reactions would result in different products.
E
NuHH E
The exceptional stabilization for the aromatic compounds leads only to the substitution (addition elimination) product.
-
21
Electrophilic Aromatic Substitution
Halogenation:1st step is generating an electrophile:
Br Br + FeBr3 FeBr3 Br Brδ+δ−
FeBr3 is a Lewis acid that coordinates with Br2 andpolarizes it, generating an electrophilic Br+
Η
Br
Η
+ FeBr3
Br Br + HBr
+ FeBr3
-
22
Electrophilic Aromatic Substitution
Nitration:
1st step is generating an electrophile, the nitronium ion:
HO NO2 + H OSO3H HSO4-
+ HO NO2
H
NO2+ + H2O
-
23
Electrophilic Aromatic Substitution
NO2+HNO2
H+HSO4
-
NO2
The highly electrophilic nitronium ion attacks the aromatic ring.
-
24
Aromatic Sulfonation
Substitution of H by SO3 (sulfonation)Reaction with a mixture of sulfuric acid and SO3 (“Fuming H2SO4)Reactive species is sulfur trioxide or its conjugate acid
-
25
Preparation of Phenols
From aromatic sulfonic acids with NaOH at high temperature
-
26
Alkylation of Aromatic Rings: The Friedel–Crafts Reaction
Alkylation among most useful electrophilic aromatic substitution reactions
Aromatic substitution of R+ for H+Aluminum chloride promotes the formation of the carbocation
-
27
Friedel-Crafts Acylationacyl group, ⎯COR
Similar to alkylation Reactive electrophile: resonance-stabilized acyl cation
-
28
Substituent Effects in Aromatic RingsSubstituents can cause a compound to be (much) more or (much) less reactive than benzene
Substituents affect the orientation of the reaction – the positional relationship is controlled
ortho- and para-directing activators, ortho- and para-directing deactivators, and meta-directing deactivators.
-
29
Ring-Activating and Ring Deactivating Substituents
Monosubstituted benzenes undergo electrophilic aromatic substitution.Substituents influence reactivity.Substituents determine position of second electrophilic substitution.Substituents that increase electron density increase reactivity.Substituents that decrease electron density decrease reactivity.
-
30
Classification of SubstituentsActivating Substituents
(ortho/para directing):• prim. Amines• sec. Amines • tertiary amines• phenolic OH• phenol ethers• amides• phenolic esters• alkyl substituents• halides
Strongly activating
weakly activating
deactivating
N H 2N H R
N R 2O H
O R
N H C R 'O
O C R 'O
RX
-
31
Classification of SubstituentsDeactivating Substituents (meta directing):
• aldehydes
• esters
• carboxylic acid
• nitrile
• sulfonic acid
• nitro
• ammonium
weakly deactivating
strongly deactivating
CHO
δ+
CORO
δ+
COHO
δ+
C N
SO3H
NO
O
NR3
δ+
δ+
-
32
Substituent Effects in Aromatic Rings
Substituents can cause a compound to be (much) more or (much) less reactive than benzene
Substituents affect the orientation of the reaction – the positional relationship is controlledortho- and para-directing activators, ortho- and para-directing deactivators, and meta-directing deactivators
-
33
Origins of Substituent Effects
An interplay of inductive effects and resonance effectsInductive effect - withdrawal or donation of electrons through a σ bondResonance effect - withdrawal or donation of electrons through a π bond due to the overlap of a p orbital on the substituent with a p orbital on the aromatic ring
-
34
Inductive Effects
Controlled by electronegativity and the polarity of bonds in functional groupsHalogens, C=O, CN, and NO2 withdraw electrons through σ bond connected to ring
Alkyl groups donate electrons
-
35
Resonance Effects – Electron Withdrawal
C=O, CN, NO2 substituents withdraw electrons from the aromatic ring by resonanceπ electrons flow from the rings to the substituents
-
36
Resonance Effects – Electron Donation
Halogen, OH, alkoxyl (OR), and amino substituents donate electronsπ electrons flow from the substituents to the ringEffect is greatest at ortho and para
-
37
An Explanation of Substituent Effects
Activating groups donate electrons to the ring, stabilizing the intermediate (carbocation)Deactivating groups withdraw electrons from the ring, destabilizing the intermediate
-
38
Halobenzenes
Cl EN= 3.5
EN= 2.5 +
Inductive effect lowers electrondensity in the ring system.
Effects act in opposite directions;inductive effect stronger.
-
39
Ammoniumbenzenes
NRR
R +
Nitrogen lacks lone pair; no resonance effect.Compare to anilines.
Positively charged nitrogenhas strong polarizing effect,lowering electron density inring system.
anilinium cation
-
40
Activating ortho/para
OH3C
o
m
p
OCH3HE
OCH3
EH
OCH3
HE
OCH3E
H
OCH3
EH
OCH3
HE
EOCH3H
OCH3
EH
H
OCH3
E
OCH3HE
OCH3
HE
There are additionalresonance contributorsfor cations inortho and parasubstitution.
These positions have adouble advantage:bigger electron densitybetter cation stabilization
-
41
Deactivating meta
NO O
H
E
NO O
NH
E
O ON
H
E
O O
NO O
EH
NO O
EH
NO
EH
O
N
E H
O ON
E H
O ON
E H
O O
There are resonance contributors for cations in ortho and parasubstitution that are lessstable because of twoneighboring +ve. charges.
-
42
Summary Table: Effect of Substituents in Aromatic Substitution
-
43
Arrange the following groups?
A) -OCH3 B) CH3C C) CO
_ _ N D) NR3+_
-
44
Trisubstituted Benzenes: Additivity of Effects
If the directing effects of the two groups are the same, the result is additive
-
45
Substituents with Opposite Effects
If the directing effects of two groups oppose each other, the more powerful activating group decides the principal outcomeOften gives mixtures of products
-
46
Meta-Disubstituted Positions Are UnreactiveThe reaction site is too hinderedTo make aromatic rings with three adjacent substituents, it is best to start with an ortho-disubstituted compound
-
47
What is the major organic product?
CH3 CH3
CH3
CH3
COCH2
O
COCH2
O
COCH2
O
(D)(C)
(B)COCH2
O
(A)
CH3Cl, AlCl3COCH2
O
1 equivalent
-
48
What is the suitable reactant to produce the following product?
AlCl3?
-
49
What is the major organic product?
1)CH3COCl,AlCl3
2) Br2, FeBr3?
-
50
Synthesis it from benzene?
CH3ClCl
NO2
OHO2N
-
51
EX: Draw the four major resonance structures of the intermediate in the reaction of anisole with HNO3/H2SO4?
OCH3
HNO3/H2SO4
OCH3
NO2
-
52
Provide the structure of the major monoitration product of the compounds below.
-
53
CN
+ Br2FeBr3
CN CN CN CN
Br Br Br Br
Br
A B C D
Chapter 4�Aromatic compoundsThe Resonance Energy of Benzene Electrophilic Aromatic SubstitutionMechanismMechanismMechanismMechanismElectrophilic Aromatic SubstitutionElectrophilic Aromatic SubstitutionElectrophilic Aromatic SubstitutionAromatic SulfonationPreparation of PhenolsAlkylation of Aromatic Rings: The Friedel–Crafts Reaction Friedel-Crafts AcylationSubstituent Effects in Aromatic Rings Ring-Activating and Ring Deactivating SubstituentsClassification of SubstituentsClassification of SubstituentsSubstituent Effects in Aromatic Rings Origins of Substituent EffectsInductive EffectsResonance Effects – Electron WithdrawalResonance Effects – Electron DonationAn Explanation of Substituent Effects HalobenzenesAmmoniumbenzenesActivating ortho/paraDeactivating metaSummary Table: Effect of Substituents in Aromatic Substitution Arrange the following groups?Trisubstituted Benzenes: Additivity of Effects Substituents with Opposite EffectsMeta-Disubstituted Positions Are UnreactiveWhat is the major organic product?What is the suitable reactant to produce the following product? What is the major organic product? Synthesis it from benzene?











![Chemistry of C-C π-bonds Lectures 5-8: Aromatic …€œOrganic Chemistry”, Clayden, Greeves, Wothers and Warren, OUP, 2000. Chapter 22 [2]. “Aromatic Chemistry” by Malcolm](https://static.fdocument.org/doc/165x107/5ad8e0b07f8b9a32618e1e06/chemistry-of-c-c-bonds-lectures-5-8-aromatic-organic-chemistry-clayden.jpg)






