Chapter 10 Review. What is the heat in Joules required to melt 25 grams of ice? Useful information:...
-
Upload
buddy-quinn -
Category
Documents
-
view
227 -
download
1
Transcript of Chapter 10 Review. What is the heat in Joules required to melt 25 grams of ice? Useful information:...

Chapter 10 Review

What is the heat in Joules required to melt 25 grams of ice? Useful information: heat of fusion of water = 334 J/g
q = m·ΔHf
• q = (25 g)x(334 J/g)• q = 8350 J

How long does it take this sample to become completely melted?
•About 9 minutes

Convert 705 Celsius to Kelvin
•705 + 273 = 978

When a solid goes directly to a gas without becoming a liquid what is it called?
•Sublimation

Absolute zero is what temperature
•0 Kelvin

The specific heat (c) of liquid water is 4.2 J/g 0C. Calculate the heat necessary to change 22 g of water (l) at 50C to 22 g of water (l) at 950C.
•Q = mcT = (22)(4.2)(90) = 8316 J

Which 2 types of change require the input of thermal energy?
•melting, vaporizing (evaporating)

This state of matter is ionized gas and the most common form of matter in the universe
•Plasma

At 80 C the substance is in what state of matter?
•liquid

The average kinetic energy of molecules in a system is called
•Temperature

WHat can be said of the temperature of a substance as it undergoes a change of state
•It remains the same

Calculate the heat needed to change a 22 g ice cube, at 00C, into 22 g of liquid at 0ºC? Heat of fusion (Hf) = 340 J/g
•Q = mHf = (22)(340) = 7480 J

When a substance is heated what happens to the average kinetic energy of the material?
•It increases

In the diagram below, at what point is the sample completely vaporized?
•At E

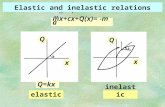
What is true about the kinetic energy in elastic collisions?
•It is conserved (it stays the same)

What is the triple point in a phase change diagram?
•The temperature point at which all three states of matter coexist

What can be said about the speed of hydrogen molecules versus nitrogen molecules if both are at the same temperature?
•Hydrogen molecules move faster

What is the arrangement of molecules in a solid called ?
•crystal lattice

The random motion of particles is called what?
•Brownian motion

What part of the graph shows the substance melting?
•BC

What does atmospheric pressure do as you go up in elevation ?
•decreases

The process of molecules moving from high to low concentration is called what ?
•diffusion

Why is glass called an amorphous solid?
•It does not have a crystal lattice structure

Why are gases mostly empty space?
•there are no attractive or repulsive forces between the gas molecules

Calculate the heat necessary to change 5 g of water (l) at 1000C to 5 g of vapor at 1000C. The heat of vaporization is 2260 J/g
•(5g)(2260J/g) = 22600 J

What is the triple point in the diagram?
•50

The arrow represents what process?
•sublimation

Convert 243 K to C
•273 - 243= -30 K

The specific heat (c) of solid water is 2.1 J/g 0C. Calculate the heat necessary to change 45.5 g of water (s) at -100C to 21.7 g of water (s) at 00C.
•(45.5g)(2.1J/g)(10) = 945 J

The kinetic molecular theory describes the behavior of matter in terms of particles in motion . T or F
•T

Give an example of an amorphous solid
•Wax

When don’t gases behave like ideal gases?
•At extreme temperatures