An Update on Oral Hypoglycemic Agents -...
Transcript of An Update on Oral Hypoglycemic Agents -...
An Update on Oral Hypoglycemic Agents
Dr Foo Joo Pin Consultant Endocrinologist
Specialist Endocrine Clinic
For Diabetes, Thyroid and Hormones CAMDEN MEDICAL CENTRE
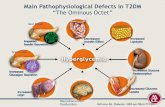
Pathogenesis of Type 2 Diabetes
HGP=hepatic glucose production.
Islet β-cell
Impaired Insulin Secretion
Increased HGP
Decreased Glucose Uptake
Increased Insulin Resistance
The Ominous Octet
Islet β-cell
Impaired Insulin Secretion
Neurotransmitter Dysfunction
Decreased Glucose Uptake
Islet α-cell
Increased Glucagon Secretion
Increased Lipolysis
Increased Glucose Reabsorption
Increased HGP
Decreased Incretin Effect
Diabetes Medications
1960 1995 2000 2005 2010
Insulin 1922
SUs 1946 / Metformin
1957
AGIs 1995
Glinides TZDs 1997
GLP1-A Exenatide Pramlintide
2005
DPP4-I Sitagliptin
2006
Liraglutide 2010
Patlak M. Breakthroughs in Bioscience 2002. http://www.faseb.org/Portals/0/PDFs/opa/diabetes.pdf Philippe J. Int J Clin Pract 2009;63:321-332
Saxagliptin 2009
SGLT2-I? Jan 2012
β-Cell Function Continues to Decline Regardless of Intervention in T2DM
T2DM=type 2 diabetes mellitus *β-cell function measured by homeostasis model assessment (HOMA) Adapted from UKPDS Group. Diabetes. 1995; 44: 1249–1258.
0
20
40
60
80
100
–5 –4 –3 –2 –1 0 1 2 3 4 5 6
Years Since Diagnosis
β-C
ell F
unct
ion
(%)*
Progressive loss of β-cell function occurs prior to diagnosis
Metformin (n=159)
Diet (n=110)
Sulfonylurea (n=511)
Case 1
• 54 year old male, BMI: 28 • Hypertension, hyperlipidemia • No history of cardiovascular event • Routine health screen
– FPG 8.6 mmol/L – OGTT: 2hr glucose 14.4 mmol/L
• HbA1c: 8.5% • Liver, renal function normal • What will you do?
Nathan et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm. Diabetes Care 2006; 31:173-5.
Biguanides
• Metformin, Metformin XR (500-1000 g ON) • Reduce hepatic gluconeogenesis – activate Adenosine
Monophosphate protein Kinase (intracellular energy sensor)
• Increased insulin-mediated muscle glucose uptake
• Decrease fatty acid oxidation – reduce energy supply for gluconeogenesis
• Non-glycemic effect – loss of appetite, lower fasting hyperinsulinemia, facilitate weight loss
• Possibly best OHGA for CV risk reduction – Selvin E, Bolen S, Hsin-Chieh Yeh HC, et al. Cardiovascular outcomes in trials of oral diabetes
medications: A systematic review. Arch Intern Med. 2008;168:2070-2080.
Biguanides - Metformin
• Risk of Lactic Acidosis < 1 per 100000 (Very rare)
• Caution in Renal Failure
– CCT of < 30 ml/min/1.73 m – contraindicated – CCT of 30-60 ml/min/1.73m – use with caution – CCT of > 60 ml/min/1.73m – safe Establishing pragmatic estimated GFR thresholds to guide metformin prescribing.Shaw JS; Wilmot
RL; Kilpatrick ES Diabet Med. 2007 Oct;24(10):1160-3. Epub 2007 Aug 2.
Other Oral Agents
• Sulphonyureas • Glinides • Αlpha-glucosidase Inhibitors • TZDs • DPP IV Inhibitors • (GLP-1 Agonist) • SGLT2 Inhibitor
Sulfonylureas
• Tolbutamide, glipizide, gliclazide, glimepiride, glibenclamide
• ATP-sensitive potassium channel in plasma membrane of β-cell • SUR, Kir6.2
• Activation closes K channels → depolarization of β-cell → Ca++ influx
which promotes insulin release
• Potency of a sulfonylurea is a function of its binding affinity to the receptor: glibenclamide the most potent, tolbutamide the least
• Side Effects: hypoglycemia, weight gain
Glinides
• Repaglinide, Nateglinide
• Stimulate insulin secretion • bind to a different site within the sulfonylurea receptor (SUR1 subunit)
• Shorter circulating half-life than sulfonylureas
• Must be administered more frequently:
– 15 mins before each meal
• Weight gain, hypoglycemia – incidence somewhat lower than sulphonylureas
Alpha Glucosidase Inhibitor
• Acarbose, miglitol, voglibose
• Competitive inhibitors of small intestine brush border enzymes alpha-glucosidases -hydrolyze oligosaccharides and polysaccharides to monosaccharides
• Carbohydrate absorption and digestion are delayed and prolonged throughout the small intestine
• Reduction of the postprandial plasma glucose elevation in both type 1 and type 2 diabetes mellitus
• Glucose lowering effect modest
• GI Side effects
Thiazolidinediones
• Side Effects: weight gain and fluid retention, with peripheral edema and a twofold increased risk for congestive heart failure, hepatotoxicity (troglitazone)
• Increased fracture risk
– Meier C, Kraenzlin ME, Bodmer M, et al.:Use of thiazolidinediones and fracture risk. Arch Intern Med 168:820–825, 2008
• Risk of Cardiovascular death
– Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471, 2007
Pioglitazone and Bladder Ca
• Preclinical : bladder Ca in rats • PROactive trial : 14 vs 5 cases in pioglit • Using FDA adverse event reporting : risk of
bladder Ca higher with piog users HR 1.22 (1.05-1.43) in French Study
• June 2011 France and Germany suspend use
• EMA’s Committee for Medicinal Products : benefits outweigh risk and piog continues to be valid option for treating certain T2DM Lancet 378:1543-1545, Nov 2011
Nauck et al. Diabetologia. 1986
Incretin effect on insulin secretion
Oral glucose load Intravenous glucose infusion
Time (min) In
sulin
(mU
/l)
80
60
40
20
0 180 60 120 0
Time (min)
Insu
lin (m
U/l)
80
60
40
20
0 180 60 120 0
Incretin effect
Control subjects (n=8) People with Type 2 diabetes (n=14)
GLP-1: effects in humans
GLP-1 is secreted from L-cells of the jejunum
and ileum
That in turn…
• Stimulates glucose- dependent insulin secretion
• Suppresses glucagon secretion
• Slows gastric emptying
Long-term effects in animal models:
• Increase of β-cell mass and improved β-cell function
• Improves insulin sensitivity
• Leads to a reduction of food intake
After food ingestion…
Drucker. Curr Pharm Des. 2001 Drucker. Mol Endocrinol. 2003
Inhibition of DPP-4 Increases Active GLP-1
GLP-1 inactive
(>80% of pool)
Active GLP-1
Meal
DPP-4
Intestinal GLP-1 release
GLP-1 t½=1–2 min
DPP-4=dipeptidyl peptidase-4; GLP-1=glucagon-like peptide-1 Adapted from Rothenberg P, et al. Diabetes. 2000; 49(suppl 1): A39. Abstract 160-OR. Adapted from Deacon CF, et al. Diabetes. 1995; 44: 1126-1131.
Newer Agents - Incretin DPP4 inhibitors (PO) GLP 1 – Agonist (SC)
Sitagliptan (Januvia®), Merck & Co, 2006 FDA
Vildagliptan (Galvus®), Novartis, 2008 EU
Saxagliptin (Onglyza®), Bristol Myers, 2009 FDA
Linagliptan (Tradjenta®) Eli Lilly, 2011 FDA
Dutogliptin, Phenomix Corp, Phase III
Gemigliptin, LG Life Sciences, Korea
Alogliptin, Takeda, FDA susp
Exenatide (Byetta®), Eli Lilly, 2005
Liraglutide (Victoza®), Novo Nordisk, 2010
Exenatide LAR, Phase III
Taspoglutide, Ispen and Roche, Phase III (Sept 10 : halted due to serious hypersensit and GI)
Alibiglutide, GSK, Phase III
AVE0010
DPP4 Inhibitors Side effects
• Low risk of hypoglycaemia, Weight neutral
• ? Immune effect - Effect on other DPP4 substrates
– Increased risk of nasopharyngitis RR 1.2, UTI RR1.5, Headache RR 1.4 in meta-analysis of sitag and vilda
• Pancreatitis : 88 cases with sitagliptan (post-marketing). 5.6 cases per 1000 patient years – similar to diabetic control group. 56 cases with saxagliptan (till Sept 2011)
• Hepatic dysfunction (Vilda) uncommon check LFT 3/12ly
• Skin : Sitaglip & saxa : Hypersensitivity reports, incl anaphy/ angioed/ SJS with sita (post-marketing)
– Serious skin reactions in animals (Vilda / Saxa)
– Skin lesion in healthy adults with higher doses Vilda
GLP-1 Agonists Exenatide Liraglutide Exenatide LAR
Dose 5mcg bd, 10mcg bd
Given within 60min prior to meals
0.6 mg od, 1.2mg od, 1.8mg od (more LOW, same A1C lowering c.f 1.2mg)
No need to give in rln meals
2.0 mg once a week
Indications Monotherapy (2009)
Add-on to Met, SU, SU+Met, TZD (+- Met)
NOT with Insulin (altho studied before)
Monotherapy
Add-on to Met, SU, Met+SU, TZD+Met
NOT with insulin
A1C lowering
Greater A1C reduction cf exenatide 1.12 vs 0.79%
Greater A1C reduction cf exenatide 1.9% vs 1.5%
Side Effects
Nausea Vomiting
Lost of Weight
Pancreatitis
(similar SE / LOW to exenatide)
Benign and malignant thyroid C-cell tumors (rats
Similar LOW to exenatide Less frequent nausea c.f daily dose (26% vs 50%)
Stimulation of calcitonin release in rats (same as liraglutide, but not exenatide) ?tumorigenesis potential
Contraind Hx / FHx Medullary Thy Ca, MEN 2A/B
• Renal Glucose Reabsorption in the Proximal Tubule
1. Adapted from Bays H. Curr Med Res Opin. 2009;25(3):671-81. 2. Wright EM, et al. J Int Med. 2007;261:32-43.
27
• Glucose is freely filtered at the glomerulus and is reabsorbed via active transport mechanisms in the proximal convoluted tubule1
• Up to 180 g glucose filtered/24 h2
GLUCOSE REABSORPTION
NO GLUCOSE
Glomerulus Proximal tubule
S1 S2
S3
GLUCOSE FILTRATION
Collecting duct ~90%
~10%
Sodium Glucose Transporter 2 (SGLT2) Inhibition: A Novel Approach to Reduce Hyperglycemia
• SGLT2 inhibition decreases plasma glucose by increasing urinary glucose excretion • Canagliflozin / dapagliflozin is a potent, selective inhibitor of SGLT2
Rothenberg PL et al. Presented at European Association for the Study of Diabetes. September 20-24, 2010; Stockholm, Sweden.
• The Renal Glucose Threhold (RTG) is Increased in Subjects with Type 2 Diabetes
Polidori D et al. 2010. Abstract 2186-PO. American Diabetes Association. June 25-29, 2010; Orlando, Florida. Polidori D et al. 2010. Presented at: European Association for the Study of Diabetes. September 20-24, 2010; Stockholm, Sweden.
29
Uri
nar
y G
luco
se E
xcre
tion
(g
/day
)
0
75
100
50
150
Plasma Glucose (mmol/L)
125
25
4 6 8 16 10 14
Below RTG minimal glucosuria occurs
12
Healthy RTG
T2DM RTG
Above RTG glucosuria occurs
~13.8 mmol/L ~10 mmol/L
• Renal glucose reabsorption is increased in diabetes, which could contribute to further increasing plasma glucose levels
Case 2
• 54 year old male,BMI: 28 • Hypertension, Hyperlipidemia • No history of cardiovascular event • Known diabetes for 3 years • No microvascular complications • No history of cardiovascular event • Receiving metformin 850mg tds • HbA1C 8.4% • Renal and liver function normal • What next?
Other Oral Agents
• Sulphonyureas • Glinides • Αlpha-glucosidase Inhibitors • TZDs • DPP IV Inhibitors • (GLP-1 Agonist) • SGLT2 inhibitor
β-Cell Function Continues to Decline Regardless of Intervention in T2DM
T2DM=type 2 diabetes mellitus *β-cell function measured by homeostasis model assessment (HOMA) Adapted from UKPDS Group. Diabetes. 1995; 44: 1249–1258.
0
20
40
60
80
100
–5 –4 –3 –2 –1 0 1 2 3 4 5 6
Years Since Diagnosis
β-C
ell F
unct
ion
(%)*
Progressive loss of β-cell function occurs prior to diagnosis
Metformin (n=159)
Diet (n=110)
Sulfonylurea (n=511)
Starting Basal Insulin
• Continue oral agents at same dose • Add single bedtime insulin (10U or 0.1U/kg)
• NPH • Glargine • Detemir
• Adjust dose according to fasting glucose
• Watch for nocturnal hypoglycemia
Case 3
• 54 year old male, obese, hypertensive, type 2 diabetes • Known diabetes for 10 years • No microvascular complications • No history of cardiovascular event • Receiving metformin 850mg tds, glipizide 10mg bd
• HbA1C: 8.5%. • Liver, renal function normal
• What will be the next treatment option?
Key points of Statement EASD/ADA • Unless there are prevalent contraindications, metformin is
the optimal first-line drug. • After metformin, there are limited data to guide us.
Combination therapy with an additional 1–2 oral or injectable agents is reasonable, aiming to minimize side effects where possible.
• Ultimately, many patients will require insulin therapy alone or in combination with other agents to maintain glucose control.
• All treatment decisions, where possible, should be made in conjunction with the patient, focusing on his/her preferences, needs, and values.
“There are in fact two things, science and opinion; the former begets knowledge, the latter ignorance.”
- Hippocrates