Aldol and claisen
-
Upload
sirod-judin -
Category
Education
-
view
514 -
download
3
Transcript of Aldol and claisen

ACIDITY OF ACIDITY OF αα-HYDROGENS-HYDROGENS
ENOLS and ENOLATE IONS

C C
H
O
C C
O
C C
O
..
..
..
..:
:
::
-
-
α-hydrogen
base“enolate ion”
:B
However, theion is morenucleophilicat carbon.
acidic : pKa ~ 25
αα - - Hydrogens and Enolate IonsHydrogens and Enolate Ions
Strong bases will remove a hydrogen on the carbon next to acarbonyl group (α -hydrogen) to make a resonance stabilizedconjugate base.
NaOH, KOH, NaOR, NaH, LDA, etc.
Major resonancecontributor:charge is beston oxygen =

ACETONE ENOLATE IONACETONE ENOLATE ION
density-electrostatic potential
HOMO
..
The nucleophilic electron pair is in the HOMO
major

C C
O
C C
O
..
..
..:
::
-
-
I CH3
I CH3
Mechanisms are often drawnfrom the enolate resonance form like this:
Rather than from theketolate form like this:
Enolate Ions as NucleophilesEnolate Ions as NucleophilesMORE NUCLEOPHILIC AT CARBON, BUT BEST REPRESENTED AS THE ENOLATE
enolate
ketolate

αα -BROMINATION-BROMINATIONOF KETONESOF KETONES

BrominationBromination
O
O
Br
O
Br
Br
O
Br
Br
Br
O
Br
Br
Br
Br
KOH
Br2
O OBr Br
:
: : :::
.... ..
.. ..
..
Difficult to stop at monobromination
-
-
It just keepson going andgoing …..

IODOFORM REACTIONIODOFORM REACTION

Iodoform ReactionIodoform Reaction
R CCH3
O
R CO
O
NaCHI3
NaOH
I2+ yellow
precipitate
R CCH2
O
..
I I
R CCH2
O
I
NaOH
I2
2x more R CC
O
I
I
I
H O:..-..
R COH
O
C I
I
I
:
goodleavinggroup
-
H2ONaOH
It just keepson going andgoing …..

ALKYLATION OF KETONESALKYLATION OF KETONES
CATALYTIC BASES = NaOH, KOH, NaOR
NON-CATALYTIC BASES = NaH, LDA REACT ONCE
REACT REPEATEDLY

O
C CH3THF
O
C CH2.. _
O
C CH2 CH3
NaH
Alkylation of a KetoneAlkylation of a Ketone
α -hydrogensCH3-I
one mole one mole
+ H2
one mole
NON-CATALYTIC BASES REACT ONCE
monoalkylation
CH3-IO
C CH2.. _
LDATHF
NCHCHCH3 CH3
CH3 CH3
: :
_Li+
“LDA”Lithium Diisopropyl Amide
a strong base
Sodium Hydride
NaH

ALKYLATION OF CYCLOHEXANONEALKYLATION OF CYCLOHEXANONE
O O
O
CH3CH2 I O
CH2CH3:
::
:..
..
-
-
:..
NaOH
enolateion
O
CH2CH3CH2CH3
CH3CH2CH3CH2
difficult to stopat monoalkylationwith NaOH or KOH(catalyst, not used up)
CATALYTIC BASES REACT REPEATEDLY
It just keepson going andgoing …..

O
CH3
O
CH3CH3
O
CH3CH3
CH3
O
CH3CH3
CH3CH3
Alkylation follows the sequenceshown below.
Sequence of Alkylation - Cyclohexanone and BaseSequence of Alkylation - Cyclohexanone and Base
O
CH3
O
CH3
- -
It is difficult to stop at monoalkylationeven if one mole of CH3I is used.
This enolate haslower energy - the double bond ismore substituted.Steric hindrance is not a problem.
CH3-I This enolate hashigher energy.
O
KOH
CH3I Large or bulky groups may follow adifferent sequence than this one.
SAMESIDEFIRST

ALKYLATION OF KETONESALKYLATION OF KETONES
NON-CATALYTIC BASES = NaH, LDA

Monoalkylation is Obtained by UsingMonoalkylation is Obtained by Using “ “Non-Catalytic Bases”Non-Catalytic Bases”
A “non-catalytic base” is used up, and not regenerated.
OH
HNaH
:H O
H
O
H
OCH3
H
CH3I
+ H2
Na+
:
: : :
+ NaI
.. ..
-
-:
..
:..
-
onemole
gone
stoichiometric base

NCHCHCH3
CH3
CH3CH3
: :
_Li
+
“LDA”Lithium Diisopropyl Amide
a strong base
LDA is also a non-catalytic base. It is too strong a base to be regenerated after it is used to remove a proton from an aldehyde or ketone.
Lithium Diisopropyl AmideLithium Diisopropyl Amide
(iPr)2N (iPr)2NH..
:..- (iPr)2N
..:-
+ α -H Xdifficult
(need to add Lio)

ALKYLATION OF KETONESALKYLATION OF KETONES
ENAMINES

ALKYLATION OF CYCLOHEXANONEALKYLATION OF CYCLOHEXANONEENAMINES ALSO GIVE MONOALKYLATION
O N CH3CH2 I
O
CH2CH3
N
CH2CH2
Alkylates onceand stops !
To perform a second alkylationyou must make the enamine allover again!
+ I-
..

N
CH3
N
CH3
Sequence of Alkylation: Enamine Sequence of Alkylation: Enamine
Steric hindrance
yellow areais planar
O O
CH3
O
CH3CH3
O
CH3
Difficult to go beyond dialkylationbecause of steric hindrance.
Alkylations go one-at-a-time.You must make a new enamineeach time.
firsttime
secondtime
Notice the different order ofmethylation from that with base.
This enamine is not favored.This enamine
is favored.
( pyrrolidine + CH( pyrrolidine + CH33I )I )

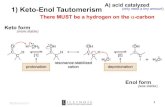
REACTIONS OF REACTIONS OF αα -HYDROGENS :-HYDROGENS : ALDOL AND CLAISENALDOL AND CLAISEN CONDENSATION REACTIONSCONDENSATION REACTIONS

C CH
O
H
C CH
OH
H
C CH
O
NuNu::B
nucleophilicaddition
.. -
removalof α-H
TYPES OF REACTIVITY FOR TYPES OF REACTIVITY FOR ALDEHYDES AND KETONESALDEHYDES AND KETONES
• Good nucleophiles add.• Strong bases remove α -hydrogens.
Often, both processes compete.

ALDOL CONDENSATIONALDOL CONDENSATION

R CH2 C H
O
R CH2 C H
OR CH2 C
OH
H
CH C H
O
R
+
R CH2 CH C C H
O
R
The Aldol CondensationThe Aldol Condensation
base
an aldol(β -hydroxyaldehyde)
ald+ol
H3O+ - H2O
α ,β -unsaturated aldehyde
aldols easily losewater to form adouble bond

CH3 C H
O: :
+ O H:.._
..
: : : :..
_.. _
CH3 C H
O: :
CH2 C H
O: :..
_
:..
:
: :
: :..
: :
+ H2O
:..
: :
CH3 C
O
H
H
+ :OH..
..
_
CH2 C H
O
CH2 C H
O
CH2 C H
O
CH2 C H
O
_
_
CH3 C
O
H
CH2 C H
O
CH3 C
O
H
Aldol Condensation -- MechanismAldol Condensation -- Mechanism
fast
fast
slow
enolate ion
forms new C-C bond

+ _CH3 C
H
O_
CH3 C
H
O
CH2
C
H
O
.._
CH2
C
H
O
The Bond Forming StepThe Bond Forming Step
nucleophile(donor)
carbonyl(acceptor)
enolate

Ketones Also Give Aldol CondensationsKetones Also Give Aldol Condensations
C
O
CH3C
OH
CH3CH2C OCH2
C O
..NaOH
C CH3CH
C O
“aldol”
-H2O
-

““CROSSED” ALDOLCROSSED” ALDOL CONDENSATIONSCONDENSATIONS

Crossed Aldol CondensationsCrossed Aldol CondensationsKETONE + ALDEHYDE
C
O
H C
OH
H
CH2C OCH2
C O
CH CH C
O
..
a “chalcone”
- H2O
NaOH
Works best to if an aldehyde isthe “acceptor”, since they aremore reactive; and works reallywell if the aldehyde has no α-H.
The ketone should have the α-H.
aldehyde
ketone
-

IMPORTANT GUIDELINESIMPORTANT GUIDELINES
Aldehyde carbonyl groups are more reactive towardnucleophilic addition than ketone carbonyl groups.
O
CR H
O
CR Rδ+
δ-δ+
δ-+I +I
+I
Nu:
MOREREACTIVE
Alkyl groupsdeactivate thecarbonyl ( +I )to addition.
1.

H-C-H CH3-C-H CH3-C-CH3
O O O
RELATIVE REACTIVITY OF C=O GROUPSRELATIVE REACTIVITY OF C=O GROUPS
Density - LUMO plots ( color scale 0.000 to 0.030 )
MOREREACTIVE
LESSREACTIVE
THE EFFECT OF ALKYL SUBSTITUTION

Ketones form enolate ions more easily than aldehydes.
O
CC HR
RO
CC RR
R
- -:: ::.. ..
More substituentson the double bond
more stable
Which enolate willform fastest?
..
..
..: :
..: :
..:
..:
-
-
-
-CH3CH2 C
O
CH3
CH3CH2 C
O
CH2 CH3CH2 C
O
CH2
CH3CH C
O
CH3 C
O
CH3CHCH3
disubstituted
monosubstituted
2.
aldehyde enolate ketone enolate

In “mixed” reactions the ketone enolate usually adds to the aldehyde.
ALDEHYDE + KETONE ?ALDEHYDE + KETONE ?
The ketone forms the lower energy enolate (forms faster)
and it adds to the aldehyde (more reactive C=O).

WHAT ABOUTWHAT ABOUTTWO DIFFERENT KETONES ?TWO DIFFERENT KETONES ?

HOW MANY PRODUCTS WITH THIS ONE ?HOW MANY PRODUCTS WITH THIS ONE ?
CH3 C
O
CH2 C
O
CH3CH3CH2+
x2 x2
8 POSSIBLE PRODUCTS !
two different self dimers two different self dimers
four mixed products
A B
a b c d
aB, bB, cA, dA
aA, bA cB, dB
….. which enolate do you think will form preferentially?

FORMATION OF RINGSFORMATION OF RINGS

Formation of RingsFormation of Rings
O
CH3
O
CH2
CH3
O
OH
O
CH3
CH3 C
O
CH2CH2CH2 C
O
CH3
NaOH
:-
α1 α2
Why don’t α2 hydrogens react ?

CH2PhC
CH2
O
Ph
O O
PhPh
O
PhPh
Ph Ph
KOH
EtOH
TETRAPHENYLCYCLOPENTADIENONETETRAPHENYLCYCLOPENTADIENONE

O
OH
H
OH
OO
1) O3
2) H3O+
KOHAldol
H2SO4
- H2O
OH-
An Interesting SequenceAn Interesting Sequence

CLAISEN CONDENSATIONSCLAISEN CONDENSATIONS

CH2 C O CH2
O
R CH3
CH2 C O CH2
O
R CH3
CH2 C
O
R CH C
R
O
O CH2 CH3
+ NaOCH2CH3
+
CH3 CH2 O H
The Claisen Ester CondensationThe Claisen Ester Condensation
a β -ketoester
CH3CH2OH
Notice that the base, the solvent and the leaving group
CH3CH2O- Na+, CH3CH2OH, CH3CH2O
-
all match (this is required in most cases).

1)
2)
+
:..
..
CH3 C OC2H5
O
O C2H5
_ :_ : :
.. _
CH3 C OC2H5
O
:_
CH2 C OC2H5
O
: :.. _
CH2 C OC2H5
O
CH3 C OC2H5
O
CH2 C OC2H5
O
CH2 C
O
OC2H5
Claisen Ester Condensation MechanismClaisen Ester Condensation Mechanism
3)
: :.. _
CH3 C
O
CH2 C OC2H5
O
: :
+ O C2H5:
..
..
_
CH2 C OC2H5
O
CH3 C OC2H5
O

Dieckmann CondensationDieckmann Condensation
C
CH
O
O
CH3
C O
O
CH3
O
C
O
O CH3
CH2CH2CH2CH2 COOMeMeOOC
..
A CYCLIC CLAISEN CONDENSATION
NaOMe
MeOH

PATTERNSPATTERNS
R CH2 C
OH
R
CH C R
O
R
R CH2 C C C R
O
RR
3-hydroxyaldehyde or
3-hydroxyketone(H)
(H)
β-hydroxy to C=O
α ,β -unsaturated C=O
2-propen-1-al or
2-propen-1-one
ALDOL
ALDOL
CLAISENR CH2 C
O
CH C OR
O
R
β-keto ester
Type of CondensationReaction
-H2O
(with loss of H2O)

SYNTHESISSYNTHESIS

C
CH3
OCH2
CH2
CCH2
O
CH2 CH CH CH2 CH3
C
CH3
CH2
CH2
CCH
O
CH2 CH CH CH2CH3
OHKOH
O
CH3
CH2
C C
CH2 CH3
H H
- H2O
Synthesis of a Perfumery CompoundSynthesis of a Perfumery Compound
Why don’t theother sets of α-Hreact?
cis-JasmoneScent of Jasmine in perfumes.
Aldol Condensation
Dehydration
cis cisα1α2
α3
α4

+CH2 CH C H
O
OHO
P
OH
OO
CH2 C CH2 O P OH
OH
O O
O
Glyceraldehyde-3-phosphate
CH2 CH C CH C CH2 O P OH
O
O
O
OH
OH
HOHO
P
OH
O O
Dihydroxyacetone phosphate
Fructose-1,6-diphosphate
α
ALDOLCONDENSATION
enzyme
Biological Synthesis of FructoseBiological Synthesis of Fructose

CH3 CH2 CH2 C H
O
CH3 CH2 CH2 C H
O
α
NaOH CH3 CH2 CH2 C
OH
CHCH2CH3 C H
H
O
CH3 CH2 CH2 C
OH
CHCH2CH3 C H
H
O
H2
Ni
CH3 CH2 CH2 C
OH
CHCH2CH3 C H
O
H H
H
ALDOLCONDENSATION
HYDROGENATION
2-Ethyl-3-hydroxyhexanal
2-Ethyl-1,3-hexanediol
--used in "6-12" insect repellent
Synthesis of an Insect RepellentSynthesis of an Insect Repellent
![Index [application.wiley-vch.de] · 1388 Index aldol condensation 477 – ultrasonic conditions 602 aldol cyclization 484 ... – Michael–aldol–dehydration 64 – Mukaiyama 247,](https://static.fdocument.org/doc/165x107/5f07e4047e708231d41f4542/index-1388-index-aldol-condensation-477-a-ultrasonic-conditions-602-aldol.jpg)