1 Nucleophilic reactions involving enolate anions (2) Aldehydes, Ketons and other carbonyl compounds...
-
date post
20-Dec-2015 -
Category
Documents
-
view
226 -
download
2
Transcript of 1 Nucleophilic reactions involving enolate anions (2) Aldehydes, Ketons and other carbonyl compounds...
1
Nucleophilic reactions involving enolate anions (2)
Aldehydes, Ketons and other carbonyl compounds having H on α-C -> in equilibrium (in solution) -> Keto-Enol tautomerization
2
Nucleophilic reactions involving enolate anions
Acylation of enolate anions -> Claisen reaction (condensation)
2 mol ester - (base) -> β-ketoester
3
Nucleophilic reactions involving enolate anions
Acylation of enolate anions -> Claisen reaction (condensation)
OEt- -> is strong base rather than good leaving groupe
reaction will run further -> anolate anion produced ->
acid required to regenerate β-ketoester
H+ /acid
If on α-C just one H -> no reaction under this condition
-> no α-H left to produce anolate anion resonance structure
4
Nucleophilic reactions involving enolate anions
Acylation of enolate anions -> Claisen reaction (condensation)
Claisen and aldol in nature -> Cholesterol biosynthesis
5
Nucleophilic reactions involving enolate anions
Intramolecular Claisen reaction -> Dieckman reaction
7
Nucleophilic reactions involving enolate anions
Mixed Claisen reaction
Better synthesis approach !!!
8
Nucleophilic reactions involving enolate anions
Aldol - Claisen reaction -> prediction of product
Aldol -> Keton is electrophile
Claisen -> Ester is electrophile
9
Nucleophilic reactions involving enolate anions
Aldol - Claisen reaction -> prediction of product
Aldol + Claisen reaction are in equilibria
-> disturbing the equilibria -> product formation
1. Dehydration in aldol (slide 14)
2. Ionization in Claisen (slide 27)
-> Ionization determinant -> Claisen reaction occurs
Product from Claisen gives the more acidic product
10
Nucleophilic reactions involving enolate anions
Reverse Claisen reaction
Driving force for Claisen reaction -> formation of enolate anion of the β-ketoester product (ionization)
If they cannot be formed -> reverse reaction controls equilibrium
11
Nucleophilic reactions involving enolate anions
Decarboxylation reactions
β-ketoesters + acid catalysed -> β-ketoacid -> loss of carbon dioxide -> decarboxylated
12
Nucleophilic reactions involving enolate anions
Decarboxylation reactions
β-ketoesters + acid catalysed -> β-ketoacid -> loss of carbon dioxide -> decarboxylated
β-ketoesters are intermediates to obtain substituted ketons
13
Nucleophilic reactions involving enolate anions
Decarboxylation reactions
β-ketoesters + acid catalysed -> β-ketoacid -> loss of carbon dioxide -> decarboxylated
β-ketoesters are intermediates to obtain substituted ketons
16
Nucleophilic reactions involving enolate anions
Nucleophilic addition to conjugated systems
1,2 addition versus 1,4 addititon:
-> Nu good leaving group -> 1,2 addition is reversible -> 1,4 product (thermodynamic control)
-> Nu bad leaving group -> 1,2 addition irreversible -> 1,2 product (kinetic control)
-> stereochemistry also important -> large Nu –> 1,4 addition preferred
Except -> Grignard + LiAlH4 hydration -> 1,2 addition
17
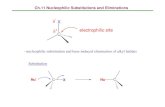
Nucleophilic reactions involving enolate anions
Nucleophilic addition to conjugated systems
Grignard
LiAlH4 Hydration
18
Nucleophilic reactions involving enolate anions
Nucleophilic addition to conjugated systems – Michael reactions
Enolate anion as nucleophile
Production of Steroid hormones (Testosterone male sex hormone)
19
Nucleophilic reactions involving enolate anions
Nucleophilic addition to conjugated systems – Michael reactions
Michael acceptors can be carcinogenic
Michael acceptors can also be utilized by the human body


































![Transannular Cyclization of Dehydrobenzo[12]annulene Induced by Nucleophilic Attack Tobe Lab Ayumi Yoshizaki 1.](https://static.fdocument.org/doc/165x107/56649cd75503460f9499f67b/transannular-cyclization-of-dehydrobenzo12annulene-induced-by-nucleophilic.jpg)



