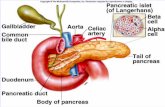
What actually happens inside the cell in response to genetic engineering, not just how we manipulate...
-
Upload
cuthbert-anderson -
Category
Documents
-
view
224 -
download
0
Transcript of What actually happens inside the cell in response to genetic engineering, not just how we manipulate...
What actually happens inside the cell in response to genetic engineering, not just how we manipulate and alter cell
Can use to predict responses of the cell Preemptive preparation against
negative response Different induction system
Heat- inducible expression system pros:
- λ pL/pR system relies on a strong and finely regulated promoter
- No special media or toxic chem. Inducers
- Culture handling and contaminations risks low
- Easily scalable (culture volume)
- Yield up to 30% recombinant protein (RP)/ total cell protein
• Perfection?
Chemical inducers (eg. IPTG):-expensive -toxic-Possible additional controls to remove chemicals (esp . for human use!)
Systems based on nutrient exhaustion: (eg. Depletion of an a.a.)- starvation affects cell metabolism, synthesis of the recombinant protein- Precise control of induction timing is difficult
Heat shock response (HSR) Overproduction of RP (often in T7 too) ->
heat shock like response, stringent response and a metabolic burden to the cells
Both HSR and RP overproduction-> converge on activation of genes coding for chaperones and proteases (sigma32 regulon)
Specific growth rates decrease, ribosomes degrade, central carbon metabolism altered
-> affects RP production How to avoid growth cessation, increase
productivity, improve purification of RP
cI857 mutant (1966): retains wild-type properties at low temperature, but unstable when temperature raised- Interactions of cI857 with operators released up to 37 C, > 37 C mutant repressor inactivated
1979:1st expression vectors using the pL promoter (production: 6.6% -> now 30%)
1983: increased productivity through temperature-regulated runaway replication, plasmid with cI857 high compatibility
Other improvements: synthetic RBS, suitable poly-linkers, mutation to operator oR -> tight repression up to 39 C (Helicobacter) (2005)
Similar system in l. lactis using comparative molecular modeling of the known 3D structure of cI857
Sigma32 regulon includes almost all genes for proteins involved in folding and degradation (chaperones, proteases)
Temperature increase -> nucleotide misincorporation and chromosome damage; sigma32 activation -> DNA and RNA protected by members of the regulon; other regulon members transfer delta-3-isopentyl-PP to tRNA to stabilize codon-anticodon pairing to improve tRNA thermal resistance
overexpression and accumulation of unfolded recombinant proteins -> genes involved in protein folding and degradation respond; most of these controlled by sigma32
-Initial rapid upregulation of genes for chaperons and proteases (some in minutes) -> unstable environment -> metabolic burden -> slow growth rate and quantity protein produced-High protein production -> a.a. depleted (min. media) -> deactylated tRNAs bind to ribosome -> RelA recognizes and makes alarmones (p)ppGpp -> stringent response -> higher transcription of stress-related genes and translation process interrupted-> as above-Both limit RP production
Harcum and Haddadin: dual stress of heating above 37 C and accumulation of unfolded RP (heated 50oC and IPTG-induced)
Found: 163/1881 genes responded in dual stress vs. either heated or induced
Genes coding for RNA polymerase (eg. rpoA/S) and ribosome coding genes downregulated
Decrease in specific growth rate Increase in respiration (RP production
and hsp increase ATP requirements 6x) Alteration of central carbon
metabolism, glucose consumption
Plasmid segregation Host strain Recombinant protein and localization Culture strategies Induction strategy – Heating duration
and intensity
Plasmid maintenance and replication -> metabolic load and consumption of resources (further drained upon induction of RP production) = plasmid-load
Plasmid-free cells favored at higher temperatures (derepressed).
In RP production: avoid plasmid segregation and extend the production phase after induction: maintain plasmid copy number with culture strategies
Culture modes: batch, fed-batch and continuous For plasmid copy# maintenance: fed-batch (temporal): restrict specific growth rate to
low values increasing rates of substrate addition before induction -> high cell concentrations
Continuous (spatial): higher plasmid stability and high cell density cultures in 1st , high RP productivity in 2nd (induced)
Lim and Jung: 23x final contration in fed-batch vs. batch culture (controlled substrate feed rate during growth phase and specific growth rate in production phase)
Curless et al.: 4-fold production under higher dilution rates tested – pre-induction specific growth rate affect productivity
Different e coli strains have different heterologous gene expression capacities
Protease-deficient: eg. BL21 most productive in a study
We use BL21s for expression
Thermoinduced system’s response can lead to recombinant proteins being degraded
Comparison study suggests factors: RP’s proteolytic sensitivity and thermal lability
Depending on localization signals: Aggregates in the cytoplasm –IB easily
isolated but have to refold after Soluble form in cytoplsam Soluble form in periplsamic – less
proteolytic activity, simpler purification, fewer isoforms and post-trans. modifications, in vivo cleavage of signal peptide, formation of disulfide bonds
secreted to supernatant
Heat inducible system has many advantages but stresses cell out
Dual stress triggering of chaperone and protease production leads to comprised RP production
How to optimize productivity of RP