-Peptide Chimeras as Novel Antimicrobials...piperidine/DMF. Continuing elongation of the ε-peptide...
Transcript of -Peptide Chimeras as Novel Antimicrobials...piperidine/DMF. Continuing elongation of the ε-peptide...

ε-Peptide Chimeras as Novel Antimicrobials
Yi-An Lu1, Jin-Long Yang2 and James P. Tam1
1Department of Biochemistry, The Scripps Research Institute, Jupiter, FL 33458, USA; 2Vanderbilt Eye Institute, Vanderbilt University, Nashville TN 37232, USA
Introduction The prevalence of antibiotic resistance of bacterial pathogens requires development of new antibacterials to overcome this limitation. Our previous work has demonstrated that cationic antimicrobial peptides with unusual architecture are often resistant to proteolytic degradation [1]. An example is a cascade-type of dendrimeric peptide (RLYR)4K2K that shows high in vitro antibacterial activity. It contains a trilysine core (K2K) that radiates four tetrapeptide RLYR arms with its sequence derived from the repeating topological motif of a known and potent antimicrobial peptide, protegrin-1 [2].
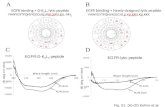
Here, we report our continuing effort in developing new antimicrobial peptides with novel branched architecture, using the ε-amine of lysine for the ε-peptide backbone and functionalized α-amines as pendant side chains to give a branched α,ε-peptide containing two RLYR motifs. Since ε-peptides are found naturally as poly-ε-lysines which are generally stable to proteolytic enzymes, but exhibit extended conformation [3], we have designed an α,ε-peptide chimera ε(RLY)-R-R-ε(YLR) with a Arg-Arg dipeptide to facilitate a reverse turn that could mimic the two-β-stranded protegrin-1 (PG-1). Our results show that the new α,ε-peptide displays broad-spectrum antimicrobial activity against Gram-positive bacteria, Gram-negative bacteria and fungi. Results and Discussion Our prototypic design of the α,ε-peptide chimera ε(RLY)-R-R-ε(YLR) was an open-chain mimetic of protegrin (Figure 1) by rearranging the architecture of the cascade-type (RLYR)4K2K to a branch-type peptide dendrimer. The chimera is symmetrical in design with two parallel extended strands of ε-peptides ε(RLY) and an α-dipeptide Arg-Arg as the reverse turn. The sequence of RLYR (basic-hydrophobic- hydrophobic-basic amino acid) [1] was derived from PG-1 (RGGRLCYCRRRFCV- CVGR) containing 18 α-amino acids that give its α-peptide backbone 54 atoms in length [2]. In contrast, the prototypic α, ε chimera, ε(RLY)-R-R-ε(YLR) with ε-lysyl peptide as the main backbone has a length of 48 atoms contributed by only 8 amino acids. The side-chain residues R, L, and Y were attached onto the α-amino ends of the ε-peptide chain to mimetic the side chain of PG-1.
The α,ε-peptide was assembled by the stepwise solid-phase method using both Boc- and Fmoc-chemistry. Boc-Bzl chemistry was used to elongate ε-peptide chain to afford an α,ε-octapeptide chimera and Fmoc-Bzl chemistry used for attaching Nα-amino acid (R, L, Y) on Nα-lysine. The synthesis of ε(RLY)-R-R-ε(YLR) was initiated on a Fmoc-Lys(Boc)-MBHA-resin, a TFA-stable benzhydrylamine resin. Functionalization of the α-amine of the Fmoc-Lys(Boc)-MBHA-resin was mediated by coupling with Ac-Arg(Tos) after removing its Nα-Fmoc protecting group by 20% piperidine/DMF. Continuing elongation of the ε-peptide chain was performed by removing Boc-group on ε-Lys, followed by coupling with Fmoc-Lys(Boc), and then a repeating deprotection/coupling cycle on the Nα-amino terminal to append the
S. Del Valle et al. (eds.), Peptides for Youth: The Proceedings of the 20th American Peptide Symposium, 393DOI: 10.1007/978-0-387-73657-0_171, © Springer Science+Business Media, LLC 2009

Y.-A. Lu et al.
commercially available Ac-Leu and Ac-Tyr(Br-Bzl) as side chains to complete the first half of the ε-peptide chimera. In the putative turn sequence, Boc-chemistry to couple two Arg in a straight forward manner, followed by strategy described above to complete the second half of the ε-peptide chimera to afford final peptide chain ε[Ac-Arg(Tos)-Ac-Leu-Ac-Tyr(Br-Bzl)]-Arg(Tos)-Arg(Tos)-ε[Ac-Tyr(Br-Bzl)-Ac-Leu-Ac-Arg(Tos)]-MBHA-resin which was cleaved by HF to give 40% yield of expected peptides after purification.
A two-stage radial diffusion assay [4] was employed to test the antimicrobial activity of ε(RLY)-R-R-ε(YLR) which shows a broad-spectrum retained activity and potency comparable to protegrin-1 under low-salt conditions.
In conclusions, this study provides insights to simplify peptidyl architectures and to learn rules for designing α,ε-peptide as β-strand mimetics found in antimicrobial peptide such as protegrins. Our solid-phase syntheses of α,ε-peptide also represent the first step toward an exploitation the potentials of α,ε-peptide as novel antibacterials. Acknowledgments The work was in part supported by US public Health Service NIH Grant EB001986. References 1. Tam, J. P., Lu, Y-A. and Yang J-L. Eur. J. Biochem. 269, 923-932 (2002). 2. Fahrner, R. L., et al. Chem. Biol. 3, 543-550 (1996). 3. Shima, S., et al. J. Antibiotics 37, 1449-1455 (1984). 4. Lehrer, R. L., et al. J. Immunol. Med. 137, 167-173 (2000).
Fmoc-Lys(Boc)-(1)
(1) 20% piperidine/DMF, coupling Ac-Arg(Tos)-OH(2) 50%TFA/DCM, coupling Fmoc-Lys(Boc)-OH (3) Repeat (1) coupling Ac-Leu-OH, then repeat (2)(4) Repeat (1) to assemble Ac-Tyr(Br-Bzl)-OH(5) Boc-chemistry coupling consecutive two Boc-Arg(Tos)-OH(7) Repeat (2), then repeat (1) to assemble Ac-Tyr(Br-Bzl)-OH(8) Repeat (1) to assemble Ac-Leu-OH, then repeat (2)(9) Repeat (1) to assemble Ac-Arg(Tos)-OH(10) HF
Ac-Arg(Tos)-Lys(Boc)-(2)
Ac-Arg(Tos)Lys-Fmoc-Lys(Boc) (3)-(10)α
ε
Arg-ArgAc-Arg
LysAc-Leu
LysAc-Tyr
LysAc-Tyr
LysAc-Leu
LysAc-Arg
Lys-CONH2H2N
Fig. 1. Synthesis of ε(RLY)-RR-ε(YLR) and its structure.
394